West Coast Retina
Case of the Month
Aug, 2014
Presented by Michael Clamp, MD
A 68 year-old woman presents with progressive vision loss
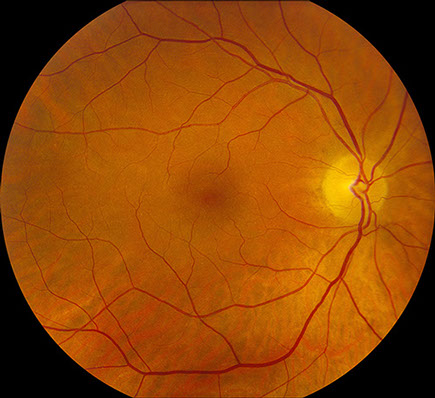
A
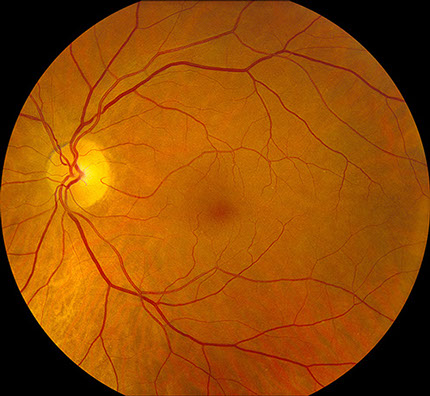
B
Figures 1A and B: Color fundus photographs of the right and left macula which appeared unremarkable.
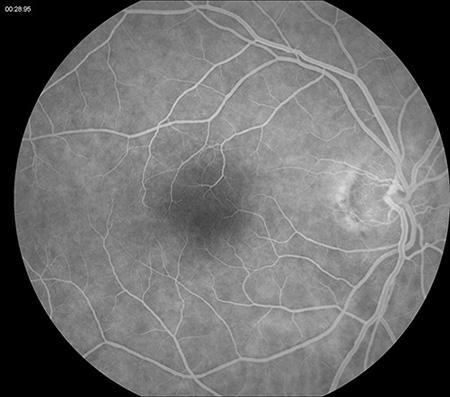
A
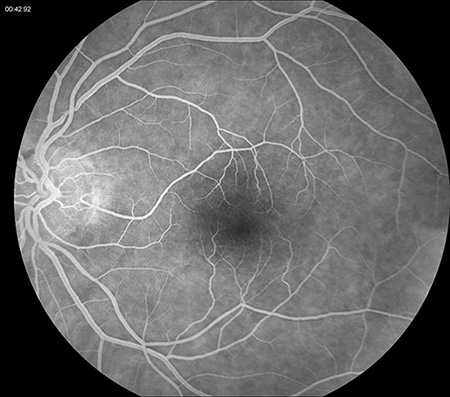
B
Figures 2A and B: Fluorescein angiogram of the right and left eye were unremarkable.
Case History
A 68 year-old woman was referred with progressive vision loss in both eyes and difficulty reading for several months. Her past medical history included Sjögren’s syndrome and osteoarthritis.
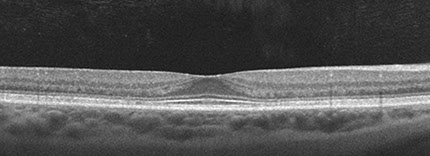
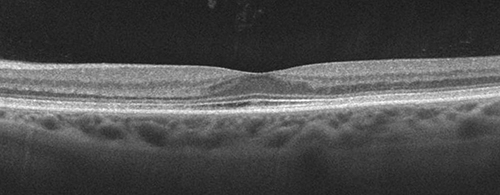
On presentation, vision in both eyes was 20/20. Intraocular pressures were normal in both eyes. Anterior segment examination revealed a quiet anterior chamber and mild cataract in both eyes. The fundus examination was unremarkable in both eyes (Figure 1). The slight grayish change seen photographically was not visible on slit lamp exam. Fluorescein angiography (Figure 2) and autofluoresence (Figure 3) did not show any significant abnormalities. Spectral-domain OCT demonstrated subtle parafoveal outer retinal changes in the left eye consistent with a bull’s-eye pattern (Figure 4).
B
A
Figures 3A and B: Fundus autofluorescence of each macula showed no obvious abnormlities.
A
B
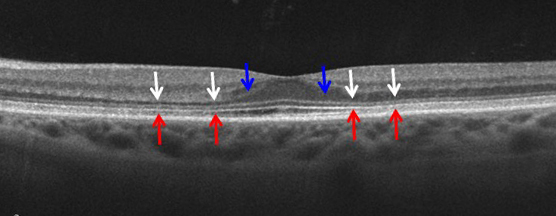
C
Figures 4A-C: Spectral domain OCT of the right (A) and left (B-C) eyes. There is loss of outer nuclear layer (ONL, white arrows) with corresponding loss of the inner segment/outer segment band (red arrows). As the ONL is lost, the orientation of Henle’s fiber layer becomes more horizontal rendering it visible (blue arrows).
What is your Diagnosis?
Differential Diagnosis
The differential diagnosis of a bull’s-eye maculopathy includes age related macular degeneration, benign concentric annular dystrophy, choroquine/hydroxychloroquine toxicity, Stargardt’s disease, central areolar choroidal atrophy, chronic macular hole, fenestrated sheen macular dystrophy, cone dystrophy, and cone-rod dystrophy. Olivopontocerebellar atrophy and ceroid lipofuscinosis can also have a bull’s-eye maculopathy, but they are associated with conspicuous systemic findings that were not present in this patient.
Additional Case History
Our patient had been taking 400mg of hydroxychloroquine daily for the past twenty years. Her daily hydroxychloroquine dose was 5.2mg/kg/day and her lifetime dose was approximately 2920 grams. These risk factors, combined with the characteristic OCT changes, led to a diagnosis of hydroxychloroquine toxicity.
Discussion
The retinal toxicity of quinolone drugs, including hydroxycloroquine (Plaquenil), was first described in the late 1960s.1 Although initially used in the treatment of malaria, hydroxychloroquine is commonly prescribed in the United States for various autoimmune conditions, including lupus and rheumatoid arthritis, and Sjögren’s syndrome. Hydroxychloroquine is relatively safe and well tolerated, however retinal toxicity can occur and is related to the cumulative dose of the medication. Patients taking less than 6.5mg/kg of body weight or up to 400mg daily and a cumulative dose less than 2920g are unlikely to develop retinopathy, but there are case reports of retinopathy in patients receiving lower dosages.
Due to the irreversible retinal toxicity of hydroxychloroquine, early detection through effective screening is critical. The earliest symptom of toxicity is usually paracentral visual field loss, for which routine Amsler grid evaluation may be helpful. However central visual acuity is often normal. The earliest ophthalmoscopic and angiographic finding is parafoveal retinal pigment epithelium (RPE) mottling. The RPE alterations can coalescence to give a bull’s eye appearance and central vision may become impaired as the RPE alteration spreads into the fovea. End stage retinopathy may appear similar to a primary tapetoretinal dystrophy with optic disc pallor and narrowing of the retinal vasculature. Fluorescein angiography and fundus autofluoresence (FAF) may demonstrate this bull’s eye pattern of RPE damage. Other clinical signs of retinopathy include paracentral visual field loss within the central 10 degrees of vision with static perimetry and decreased parafoveal receptor amplitudes with multifocal electroretinography (mfERG), but no single test has emerged as the gold standard.3, 4 The introduction of spectral domain OCT as an effective screening tool was first demonstrated by Chen et al, who described parafoveal outer retinal thinning, attenuation of the parafoveal inner/outer segment junction and loss of the normal foveal depression, which results in the characteristic OCT appearance of the flying saucer sign (Figure 5).2 Histopathologic studies demonstrate loss of the rod and cone receptor elements and subretinal clumping of pigment within the macula.6 In most cases, the retinopathy is irreversible and progresses for several years after cessation of hydroxychloroquine.
The most recent recommendations from the American Academy of Ophthalmology suggest the following screening guidelines:5 Patients being started on hydroxychloroquine should have a baseline examination performed within the 1st year of use to rule out preexisting pathology that could complicate screening or contraindicate hydroxychloroquine use. Every baseline examination should include careful biomicroscopy, automated threshold testing with a white 10-2 protocol, and, where available, testing with one or more of the recommended objective tests: SD-OCT, mfERG, or FAF. At the baseline examination the patient’s ideal body weight should be used to calculate the mg/kg dosing, but toxicity can occur even when the dose is below the well-known threshold of 6.5mg/kg/day. The most important risk factors are duration of therapy greater than 5-7 years and/or a total lifetime dose that exceeds 1000 grams. Since the drug is eliminated via both hepatic and renal pathways, liver and kidney disease are also risk factors that can contribute to unintentionally toxic dosing regimens.
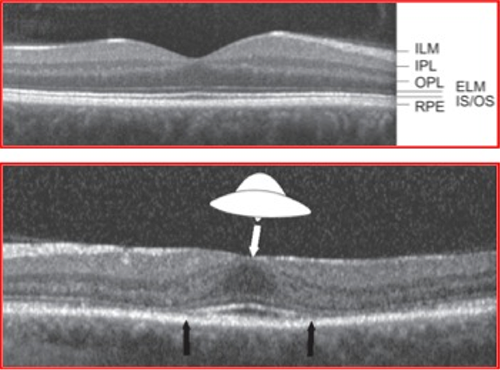
Figure 5: The flying saucer sign of Chen, et al.2
Take Home Points
- Early detection of hydroxychloroquine (Plaquenil) related retinopathy is critical to preventing irreversible vision loss.
- Screening for hydroxychloroquine retinopathy should consist of biomicroscopy, automated threshold perimetry with a white 10-2 protocol, and SD-OCT, mfERG, or FAF where available.
- Vision loss may continue for several years after cessation of hydroxychloroquine.
Want to Subscribe to Case of the Month?
References:
- Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine (plaquenil) therapy. Am J Ophthalmol. Aug 1967;64(2):245-252.
- Chen E, Brown DM, Benz MS, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the "flying saucer" sign). Clin Ophthalmol. 2010;4:1151-1158.
- Marmor MF. Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol. Apr 2012;130(4):461-469.
- Marmor MF, Melles RB. Disparity between Visual Fields and Optical Coherence Tomography in Hydroxychloroquine Retinopathy. Ophthalmology. Jun 2014;121(6):1257-1262.
- Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF, American Academy of O. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. Feb 2011;118(2):415-422.
- Wetterholm DH, Winter FC. Histopathology of chloroquine retinal toxicity. Arch Ophthalmol 1964;71:82-87.