West Coast Retina
Case of the Month
January, 2011
A 58-year-old female with bubbles in her vision.
Presented by Sandeep Randhawa, MD

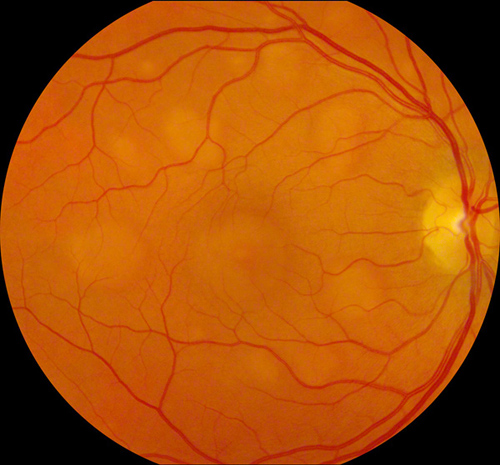
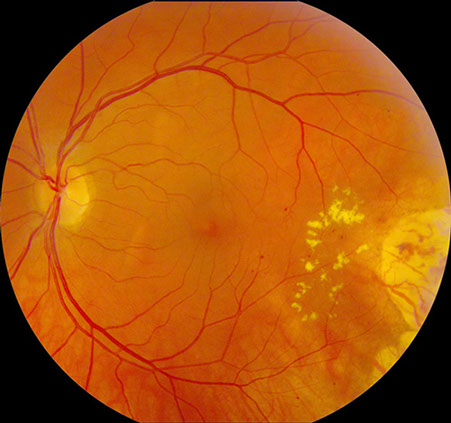
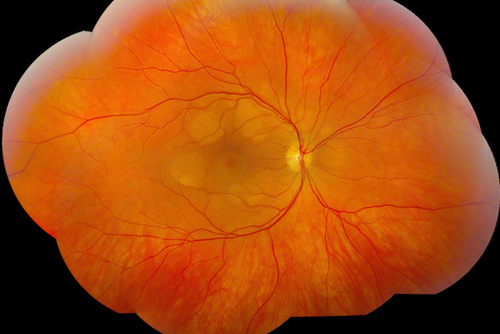
Figures 1A: Color fundus photographs of the right and left eye showing bilateral multifocal circumscribed yellow-orange vitelliform lesions in the posterior pole (more in the right eye), and the regressed choroidal melanoma in the inferotemporal periphery of the left eye with mild radiation retinopathy.
Case History
A 58-year-old female presented with a two-month history of seeing bubbles in her vision. Her past medical history was significant for choroidal melanoma in the left eye for which she had been treated with Iodine-125 plaque brachytherapy in 1991. She was found to have metastatic lung lesions in 2007 which were positive for melanoma on biopsy, and had been slow growing. She had been receiving chemotherapy (dacarbazine and dasatinib) and immunotherapy (interleukin- 2) for the metastases. She denied having any nyctalopia or photopsia. There was no family history of retinal disease.
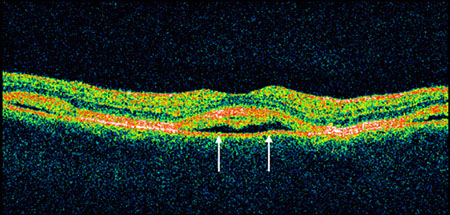
On examination, her visual acuity was 20/30 in her right eye and 20/40 in her left. The pupils, anterior segment, and intraocular pressure were normal. Dilated fundus examination revealed bilateral multifocal circumscribed yellow-orange vitelliform lesions in the posterior pole (more in the right eye), and the regressed choroidal melanoma in the inferotemporal periphery of the left eye with a few hard exudates and microaneurysms along its posterior margin consistent with radiation retinopathy (Figure 1A). Fluorescein angiography showed mild blockage of background fluorescence along the margins of the lesions in the early phase of the angiogram; no late leakage was evident (Figure 1B). The left eye showed mild late hyperfluorescence from the microaneurysms along the posterior margin of the regressed melanoma on fluorescein angiography. Optical coherence tomography (OCT) through the lesions revealed a serous neurosensory detachment in the right eye and an early neurosensory detachment in the left eye (Figure 1C).
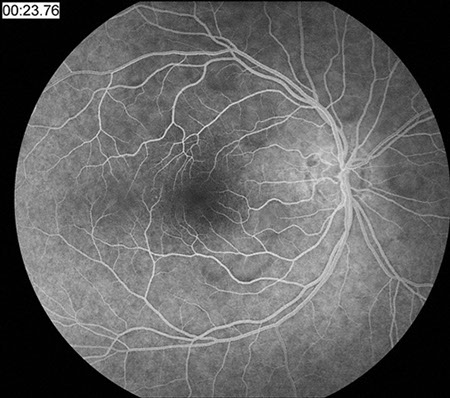
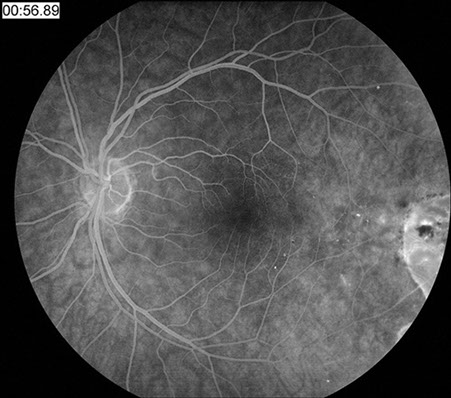
Figures 1B: Fluorescein angiography showed mild blockage of background fluorescence along the margins of the lesions in the early phase of the angiogram right eye, and mild hyperfluorescence from microanaeurysms along the posterior margin of the regressed choroidal melanoma which shows increased background fluorescence.
Right Eye
Left Eye
Figures 1C: OCT through the lesions revealed a serous neurosensory detachment in the right eye and an early neurosensory detachment in the left eye. Note the apparent deposition of material in the subretinal space in the right eye (arrows).
What is your Diagnosis?
Differential Diagnosis
A 58-year-old female with metastatic choroidal melanoma presents with bilateral, symmetric, vitelliform lesions with neurosensory detachments. The differential diagnosis includes multifocal Best’s vitelliform dystrophy, acute exudative polymorphous vitelliform maculopathy, Harada’s disease, ocular lymphoma, metastatic myeloma, idiopathic bilateral serous pigment epithelial detachments, and paraneoplastic retinopathy.
A Humphrey and Goldmann visual field test was performed and was normal. In addition, an ERG (scotopic and photopic), EOG, and serum protein electrophoresis were also within normal limits. Serum testing for anti-bipolar cells and anti retinal pigment epithelial antibodies was negative (though an atypical pattern of immunofluorescence was seen on testing on the mouse retina homogenate)
Figures 2A: Color fundus photographs (six week follow-up) showing circumscribed vitelliform lesions, with a slight pseudohypopyon appearance, more notable in the right eye.
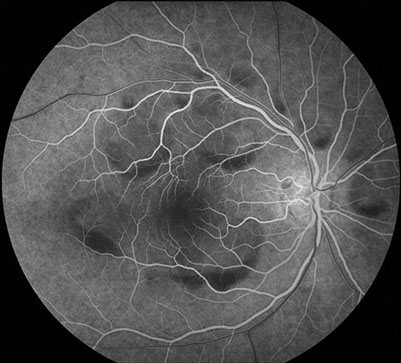
Figures 2B: Fluorescein angiography (six week follow-up) showed blockage of background fluorescence along the rim of the lesions, more prominent along an inferior crescent-shaped area, consistent with the pseudohypopyon appearance.
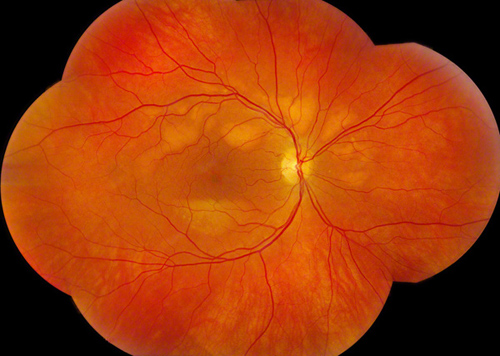
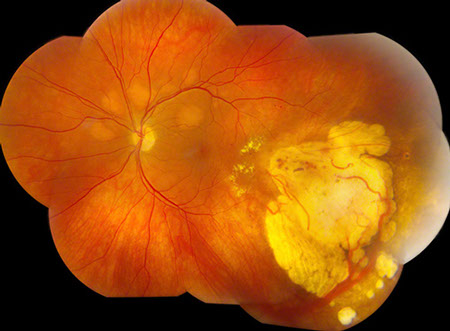
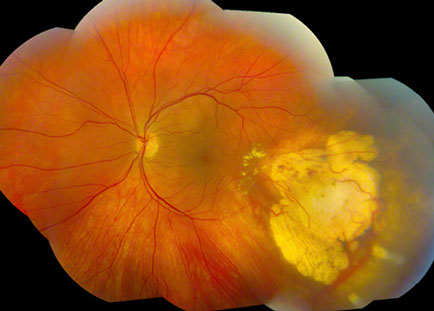
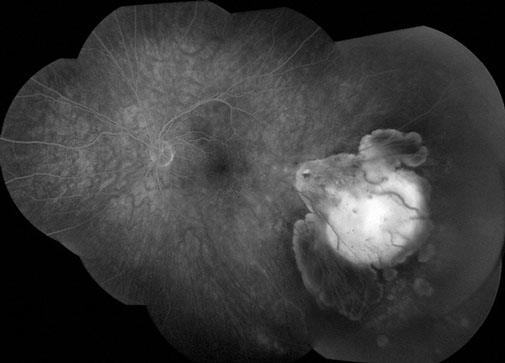
Figures 3A: Color fundus photographs (two year follow-up) revealed coalescence, consolidation and expansion of the lesions. The layering within the lesions, particularly in the right eye has become more distinct.
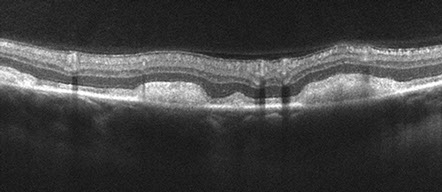
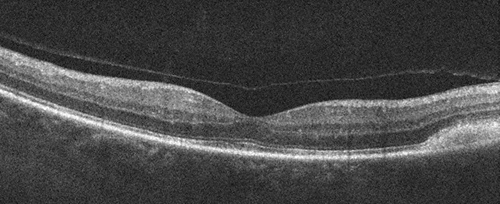
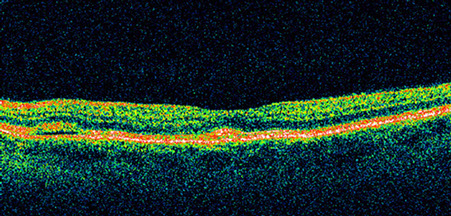
Figures 3B: Spectral domain OCT (two year follow-up) revealed bilateral multiple areas of diffuse reflectance beneath the detached neurosensory retina corresponding to the lesions. The apparent ‘serous’ fluid seen initially, appears to have resolved.
Discussion
Based on the examination, clinical course, and history, a diagnosis of paraneoplastic vitelliform retinopathy was made.
Paraneoplastic retinopathies are rare disorders which are caused by the immune system’s response to distal tumors. Autoantibodies generated against distant tumor antigens cross-react with different retinal proteins, resulting in retinal degeneration. Paraneoplastic retinopathies are usually divided into two major groups, cancer-associated retinopathy and melanoma-associated retinopathy.
Cancer-associated retinopathy is usually seen in patients with small-cell carcinoma of the lung and is associated with autoantibodies against recoverin and α-enolase.1, 2 The autoantibodies in cancer-associated retinopathy induce apoptotic death of the photoreceptors, resulting in a severe retinal degeneration affecting both cones and rods.3 Melanoma-associated retinopathy (MAR) was initially reported by Gass in an atypical case with vitelliruptive yellow retinal lesions, but most MAR cases have diffuse retinal atrophy without pigment deposits.4 Melanoma-associated retinopathy is usually seen in patients with cutaneous malignant melanoma. The disorder often appears at the stage of metastases with a sudden onset of night blindness, photopsias, shimmering, and a varying degree of visual loss.5 Melanoma-associated retinopathy has typically been associated with autoantibodies against the retinal bipolar cells, and the typical full-field electroretinogram (ERG) shows a markedly reduced or absent dark-adapted b-wave and a preserved a-wave, confirming a defect in bipolar function.6
Melanoma-associated retinopathy usually has a normal retinal appearance.7 However, more recent studies have described patients with MAR or MAR-like symptoms with posterior uveitis, pigment epithelial changes, paracentral scars, optic disc pallor, and retinal vessel attenuation.8-10 A few patients with vitelliform retinal changes or serous retinal detachments resembling Best macular dystrophy (BMD) have also been described, similar to the lesions seen in our case.11-15
In three of these patients, the primary tumor was a choroidal malignant melanoma like our case.11, 13-15 One of these cases had anti-retinal antibodies against bipolar cells, and the other two patients had MAR-like symptoms but electrophysiological and serological testing were not done, 11, 13, 14 and one had antibodies against bestrophin-1,15
More recent literature studies have reported antibodies reactive to a 22-kDa neuronal antigen,16 a 35-kDa protein in Muller cells,17 transducin-β,18 mitofilin, and titin.19 Probably several more yet unknown antigens are involved in this disorder,20 suggesting further heterogeneity of the syndrome.
Given the positive history of metastatic choroidal melanoma (in the setting of a negative family history of retinal disease), and the characteristic vitelliform lesions, our patient likely has a variant of paraneoplastic vitelliform retinopathy with antibodies to an atypical antigen (given the absence of anti-bipolar cell and anti-RPE antibodies).
Take Home Points
- Paraneoplastic retinopathies are complex disorders with several new unknown antigens being identified.
- Clinicians should consider the possibility of metastatic melanoma in patients with or without a history of melanoma who have unexplained serous retinal detachments, especially with atypical or no leakage on fluorescein angiography. The later appearance of Best’s-like subretinal deposits also may be part of the picture.
Want to Subscribe to Case of the Month?
References
- Thirkill CE, Fitzgerald P, Sergott RC, Roth AM, Tyler NK, Kaltner JL. Cancerassociated retinopathy (CAR-syndrome) with antibodies reacting with retinal, optic nerve, and cancer cells. N Engl J Med. 1989;321(23):1589-1594.
- Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancerassociated retinopathy. Clin Immunol Immunopathol. 1996;78(2):120-129.
- Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003;2(2):63-68.
- Gass J. Acute Vogt-Koyanagi-Harada-like syndrome occurring in a patient with metastatic cutaneous melanoma. In: Saari K, ed. Uveitis Update: Proceedings of the First International Symposium on Uveitis. Amsterdam, the Netherlands: Elsevier Science; 1984:407-408.
- Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol. 2001;21(3):173-187.
- Milam AH, Saari JC, Jacobson SG, et al. Autoantibodies against retinal bipolar cells in cutaneous melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34(1):91-100.
- Berson EL, Lessell S. Paraneoplastic night blindness with malignant melanoma. Am J Ophthalmol. 1988;106(3):307-311.
- Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol. 2001;21(3):173-187.
- Kellner U, Bornfeld N, Foerster H. Severe course of cutaneous melanoma associated paraneoplastic retinopathy. Br J Ophthalmol. 1995;79(8):746-752.
- Borkowski LM, Grover S, Fishman GA, Jampol LM. Retinal findings in melanoma-associated retinopathy. Am J Ophthalmol. 2001;132(2):273-275.
- Zacks DN, Pinnolis MK, Berson EL, Gragoudas ES. Melanoma-associated retinopathy and recurrent exudative retinal detachments in a patient with choroidal melanoma. Am J Ophthalmol. 2001;132(4):578-581.
- Palmowski AM, Haus AH, Pfo¨hler C, et al. Bilateral multifocal chorioretinopathy in a woman with cutaneous malignant melanoma. Arch Ophthalmol. 2002;120(12):1756-1761.
- Jampol LM, Kim HH, Bryar PJ, Shankle JB, Lee RT, Johnston RL. Multiple serous retinal detachments and subretinal deposits as the presenting signs of metastatic melanoma. Retina. 2004;24(2):320-322.
- Sotodeh M, Paridaens D, Keunen J, van Schooneveld M, Adamus G, Baarsma S. Paraneoplastic vitelliform retinopathy associated with cutaneous or uveal melanoma and metastases. Klin Monatsbl Augenheilkd. 2005;222(11):910-914.
- Eksandh L, Adamus G, Mosgrove L, Andréasson S. Autoantibodies against bestrophin in a patient with vitelliform paraneoplastic retinopathy and a metastatic choroidal malignant melanoma. Arch Ophthalmol. 2008 Mar;126(3):432-5.
- Keltner JL, Thirkill CE. The 22-kDa antigen in optic nerve and retinal diseases. J Neuroophthalmol. 1999;19(2):71-83.
- Flynn MF, Fisherman GA, Adamus G. Antiretinal Muller cell antibodies in patients with melanoma associated and autoimmune retinopathy [ARVO abstract]. Invest Ophthalmol Vis Sci. 2000;41:s567.
- Potter MJ, Adamus G, Szabo SM, Lee R, Mohaseb K, Behn D. Autoantibodies to transducin in a patient with melanoma-associated retinopathy. Am J Ophthalmol. 2002;134(1):128-130.
- Pfo¨ hler C, Preuss K-D, Tilgen W, et al. Mitofilin and titin as target antigens in melanoma-associated retinopathy. Int J Cancer. 2006;120(4):788-795.
- Ladewig G, Reinhold U, Thirkill CE, Kerber A, Tilgen W, Pfo¨hler C. Incidence of antiretinal antibodies in melanoma: screening of 77 serum samples from 51 patients with American Joint Committee on Cancer stage I-IV. Br J Dermatol. 2005;152(5):931-938.