West Coast Retina
Case of the Month
Jan, 2016
Presented by Ananda Kalevar, MD
A 44 year-old woman presents with decreased vision in both eyes.

A
B
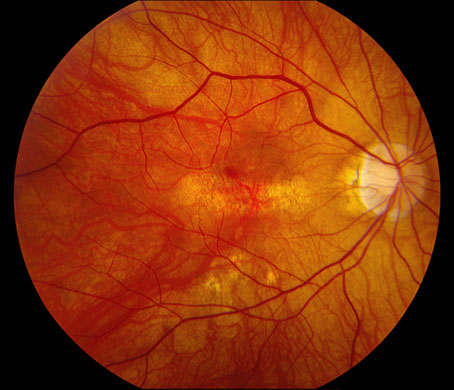
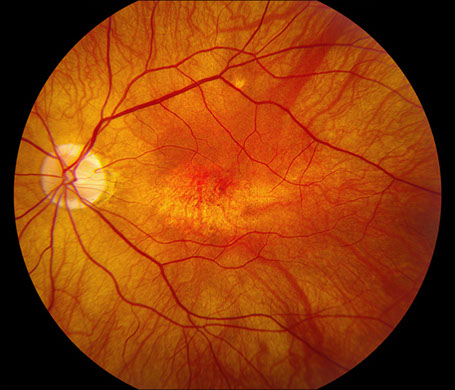
Figures 1A and B: Fundus color photographs of the right and left eye. Note the myopic appearing fundus and an ovoid area of retinal pigment epithelial mottling and atrophy in the fovea of each eye.
Case History
A 44 year-old woman presents with decreased central vision in both eyes, most notably in her left eye. Her ocular history was relevant for high myopia (-9 Diopters). Her past medical history, social history, family history and medications were non-contributory.
On examination, best-corrected visual acuity was 20/50 and 20/100 in the right and left eye, respectively. Intraocular pressure was normal in both eyes. The anterior segment examination was unremarkable. The posterior segment exam was remarkable for retinal pigment epithelium (RPE) changes with central focal RPE atrophy in both eyes (Figures 1A and B).
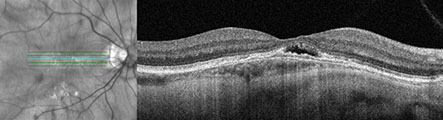
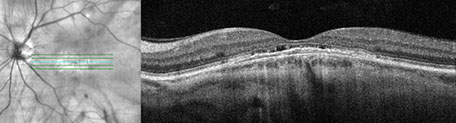
Spectral domain OCT showed a convex bulge of the choroid and retinal pigment epithelium observed on both the vertical and horizontal b-scans (Figures 2A-D). Subretinal fluid was present in each eye as well as a relatively thin choroid. Fluorescein angiography (Figures 3A and B) revealed hyperfluorescence in the fovea resulting from RPE defects but no specific leakage point. Fundus autofluorescence (Figures 4A and B) shows granular perifoveal ring of hyperautofluorescence most prominently in the right eye, and to a lesser degree in the left eye.
A
C
B
D
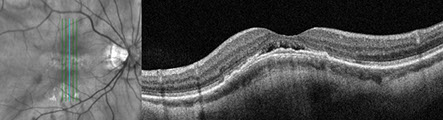
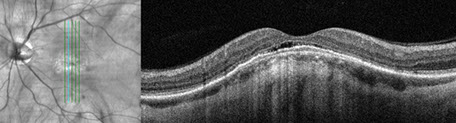
Figures 2A-D: SD-OCT scans of the right (A and B) and left (C and D). Horizontal scans through each macula (A and C) and vertical scans (B and D) reveal an convex 'bulge' of the choroidal and RPE contour, most prominently in the vertical orientation. Note the subretinal fluid present under the fovea in each eye. The choroid appears thinned centrally, and although EDI-OCT scans were not performed, the sclera appears thickened centrally.
A
B
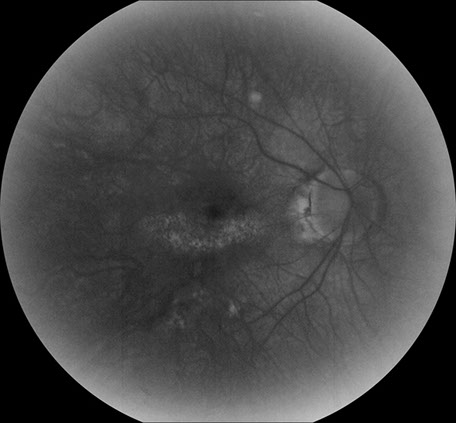
Figures 3A and B: Fluorescein angiogram of the right and left eye. Note the areas of hyperfluorescence in each macula, more prominently in the left eye than the right eye.
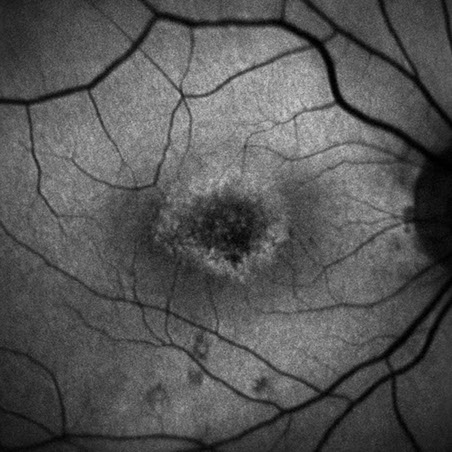
A
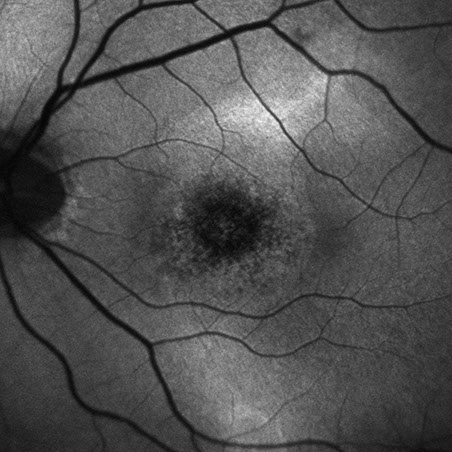
B
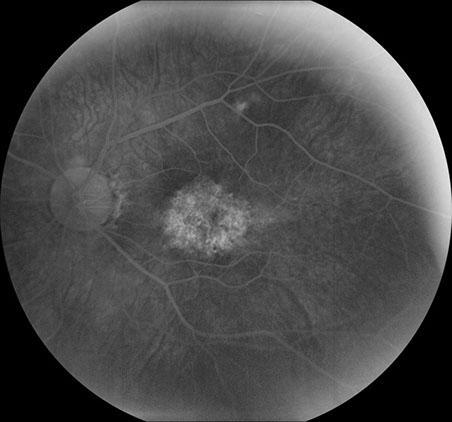
Figures 4A and B: Fundus autofluorescence scans of the right and left eye. Note the areas of increased autofluorescence in an oval pattern, most prominently in the right eye.
What is your Diagnosis?
Differential Diagnosis
The differential diagnosis in this case of myopia associated with serous foveal detachment includes: choroidal neovascularization complicating pathologic myopia, age-related macular degeneration, central serous chorioretinopathy, hypotony, orbital mass, posterior scleritis, uveal effusion syndrome and dome-shaped macula.
Discussion
Dome-shaped macula (DSM) was first described by Gaucher and coworkers in 2008 as a convex protrusion of the macula in highly myopic eyes detected by optical coherence tomography (OCT).1 Studies have shown that DSM can be found in approximately 15% of highly myopic eyes.2
Brian Curtin’s classification of posterior staphyloma conformation, done before the advent of OCT, does not include the finding of DSM.3 More recently, DSM was further classified into 3 subtypes using EDI-OCT: horizontally oriented oval-shaped domes, vertically oriented oval-shaped domes and round domes. In approximately 50% of cases, a posterior staphyloma is present. On routine clinical examination, the presence of DSM may be missed on dilated fundus examination and if OCT scans are not performed in both a vertical and horizontal manner. Approximately 75% of domes are horizontally oriented and the classic OCT scan in most clinics consists of a 5-line raster of horizontal scans. This common scan protocol would capture most of these domes, but would miss many if vertical scans were not also evaluated. However, while the diagnosis is made by OCT, three findings with ophthalmoscopy can raise suspicion of DSN: an oval area of macular pigment mottling, appearance of a ridge connecting the optic nerve and fovea, and horizontally oval optic nerve.
Myopic eyes have been shown to have a thinner choroid and sclera. In contrast, it has been demonstrated using enhanced depth imaging (EDI) and Swept- Source (SS)-OCT that relative, localized thickening of the macular sclera was present in eyes with DSM.4-6 This localized scleral thickening has been hypothesized to be the cause of this abnormality. However, it is not known why this area of scleral thickening occurs.
There are several complications that can arise with DSM including serous retinal detachment, choroidal neovascularization, retinal pigment epithelium changes, macular holes, and foveoschisis5. However, most of these are recognized complications of pathologic myopia. While the exact incidence of serous fluid associated with DSM is not clear, it does appear that this is a relatively unique occurrence associated with this entity and not secondary to associated CNV. Foveal serous retinal detachment (SRD) and choroidal neovascularization (CNV) was found to be the major cause of visual disturbances in DSM. Studies have shown that SRDs occurred in approximately ~50% and CNV in approximately 40% of eyes with DSM.
The pathogenesis of serous retinal detachment in eyes with DSM is not fully understood. It should be noted that the sclera in eyes with DSM is relatively thicker than similarly myopic eyes but still thinner than less myopic eyes. The cause of the serous fluid has been suggested to be possibly similar to that which occurs in in central serous chorioretinopathy or nanophthalmos, the latter condition being associated with a thickened sclera. Scleral thickening may impede choroidal outflow, producing choroidal thickening and subsequent serous leakage. Fluorescein angiography and Indocyanine angiography in eyes with DSM usually show pinpoint areas of hyperfluorescence, and most commonly in eyes with an associated serous retinal detachment.
There is no proven therapy for serous retinal detachments related to DSM. Intravitreal anti-VEGF injections, laser, oral spironolactone, and photodynamic therapy (PDT) have all been tried. PDT seems to have had the most success.6
It has been postulated that the presence of the dome may be protective, specifically that it may be a way the eye is attempting to compensate and reduce mechanical damage induced by myopic ocular expansion. From a surgical point of view, the DSM achieves a similar anatomic configuration as achieved following a macular buckle procedure.
Take Home Points
- Dome-shaped macula is a relatively novel diagnosis producing a convex protrusion of the macula that occurs in ~10-15% of highly myopic eyes.
- Serous retinal detachment is not uncommon and appears to be uniquely associated with DSM.
- Localized thickening of primarily the sclera and to a less degree, the choroid is noted.
- Treatment is difficult and largely unsuccessful, though PDT may offer the highest chance of success
Want to Subscribe to Case of the Month?
References
- Gaucher D, Erginay A, Lecleire-Collet A et al. Dome-Shaped Macula in Eyes with Posterior Staphyloma. Am J Ophthalmol 2008; 145: 909-914.
- Liang IC, Shimada N, Tanaka Y et al. Comparison of Clinical Features in a Highly Myopic Eyes with and without a Dome-Shaped Macula. Ophthalmology 2015; 122: 1591-1600.
- Curtin BJ. The Posterior Staphyloma of Pathologic Myopia. Trans Am Ophthalmol Soc 1977; 75: 67-86.
- Imamura Y, Iida T, Maruko I et al. Enhanced Depth Imaging Optical Coherence Tomography of the Sclera in Dome-Shaped Macula. Am J Ophthalmol 2011; 151: 297-302.
- Ohsugi H, Ikuno Y, Oshima K et al. Morphologic Characteristics of Macular Complications of a Dome-Shaped Macula Determined by Swept-Source Optical Coherence Tomography. Am J Ophthalmol 2014; 158: 162-170.
- Viola F, Dell’Arti L, Benatti E et al. Choroidal Findings in Dome-Shaped Macula in Highly Myopic Eyes: a Longitudinal Study. Am J Ophthalmol 2015; 159: 44-52.