West Coast Retina
Case of the Month
July, 2010
Presented by Jon Wender, MD
A 63 year-old female with 7 months of metamorphopsia and “swirling pinwheel” photopsias in both eyes.
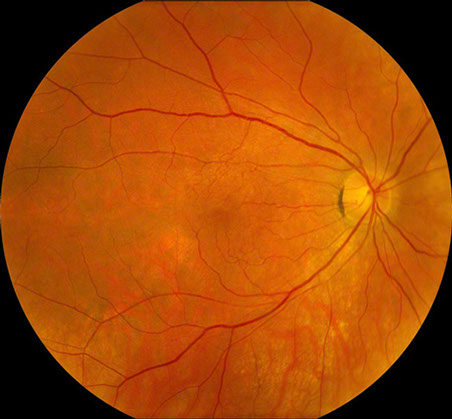
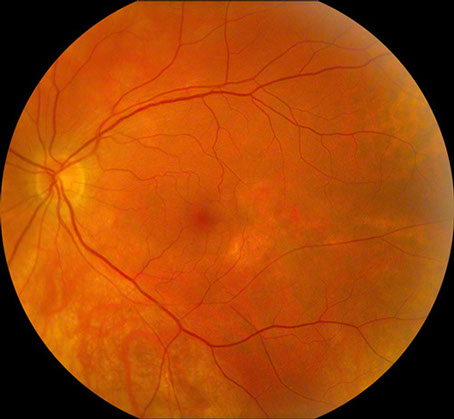
Figures 1: Color photographs of the right and left macula. Mild retinal pigment epithelial atrophy is seen inferotemporal to the right fovea, and a larger area is present temporal to the left fovea. Note the mild epiretinal membrane in the right macula.
Case History
A 63-year-old female presented with a 7-month history of metamorphopsia and “swirling pinwheel” photopsias in both eyes. Her past medical history was significant for hypertension and rheumatoid arthritis, controlled with benazepril and hydroxychloroquine (HCQ) (Plaquenil). Her regimen consisted of daily HCQ, 200 mg for 25 years. The patient weighed 36 kg with a calculated daily dosage of HCQ equivalent to 5.6 mg/kg. Due to the lack of a consistent follow up with a rheumatologist and foreign travel, she had neglected routine ophthalmic examination. She denied any family history of macular or retinal dystrophy.
At presentation, the best-corrected visual acuity was 20/16 in the right eye and 20/25 in the left eye. The intraocular pressure was within normal limits in each eye and the blood pressure was 140/80. The anterior segment examination was normal bilaterally. Dilated fundus examination revealed an epiretinal membrane in the macula of each eye as well as a subtle, arcuate area of retinal pigment epithelial (RPE) atrophy temporal to the fovea of each eye (Figure 1). Fluorescein angiography (FA) was significant for window defects that corresponded with the area of paramacular atrophy bilaterally (Figure 2).
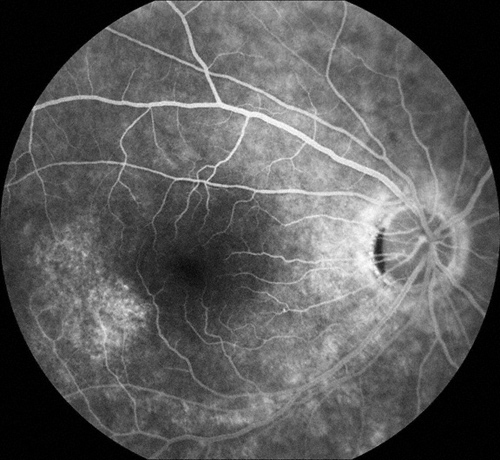
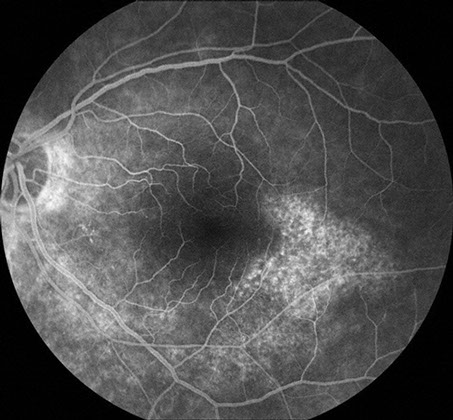
Figures 2: Arteriovenous phase fluorescein angiograms of the right and left macula. Note the areas of hyperfluorescence temporal to each macula. More subtle areas are seen in the peripapillary area and below the macula, particularly in the left eye.
What is your Diagnosis?
The history, examination, and ancillary testing were suspicious for retinal and RPE toxicity related to HCQ. Fundus autofluorescence imaging demonstrated more severe involvement than either fundus examination or fluorescein angiography (Figs 3).
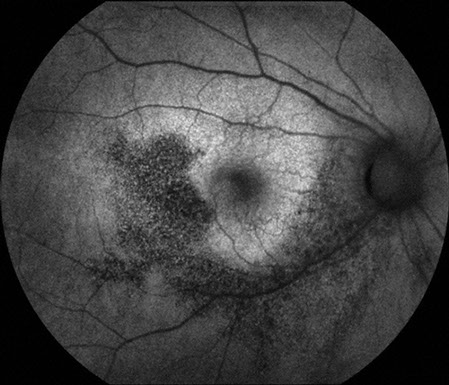
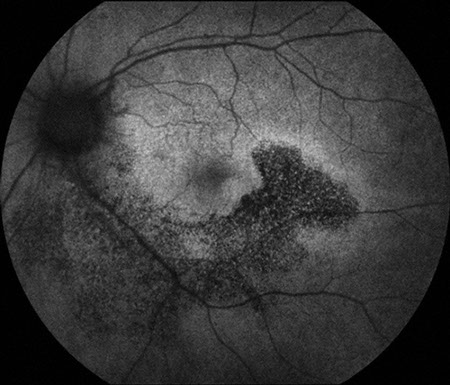
Figures 3: Fundus autofluorescence photographs of the right and left macula. Note the diminished fluorescence extending from the nerve in an arcuate fashion below each macula and temporally. The damage to the retinal pigment epithelium is much more apparent than what is imaged with color photographs and fluorescein angiography.
Discussion
Chloroquine (CQ) and hydroxychloroquine (HCQ) have been used for nearly six decades in the treatment and prophylaxis of malaria as well as in the management of systemic lupus erythematosus, rheumatoid arthritis, and other dermatologic and rheumatologic diseases.1 Both medications have an affinity for melanin-rich tissue, including the RPE, and both drugs may affect the metabolism of photoreceptors.2 The accumulation of these medications in the RPE may account for long-term toxicity, which may progress for several months after the medication has been discontinued.3 HCQ is less potent but also less toxic than CQ. The frequency of ocular toxicity in the United States has declined since HCQ has largely replaced CQ.2
The overall incidence of retinopathy associated with HCQ is low and dose-related.1 Rates of toxicity are higher among patients taking larger doses and in chronic users. Daily dosage is thought to be a more important determinant than total cumulative dose.1 The incidence of toxicity is exceedingly low (<1/1000) during the first 5 years of therapy among patients without liver or kidney disease and who are taking less than 6.5 mg/kg (based on lean body weight) per day.1 Toxicity at doses less than 6.5mg/kg/day has been reported, however, in patients taking the medication for more than 5 years.
Retinopathy caused by HCQ and CQ consists of bilateral funduscopic changes, including macular pigmentary alterations, as well as functional abnormalities, most notably, in the visual field. Symptoms may include blurred vision, photophobia, paracentral scotomas, and photopsias.1 Ophthalmoscopy may reveal granular pigmentary alterations, often in the form of a bull’s eye maculopathy, with a circle of RPE atrophy surrounding but sparing the central fovea. Standard recommended diagnostic evaluations at each screening visit consist of a complete ophthalmic examination, including visual acuity and dilated fundus examination, as well as perimetry, with either an Amsler grid or automated 10-2 Humphrey visual field (HVF).2 Optional screening modalities include fundus photography, color vision assessment, and FA. Fundus photography may be particularly useful in eyes with baseline pigmentary abnormalities, in order to establish, at future screening visits, whether progression has occurred.
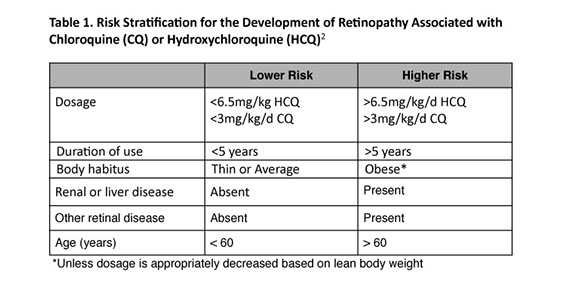
Patients taking HCQ and CQ are stratified into low and higher risk groups, depending on variables established by the American Academy of Ophthalmology (AAO) (Table 1).2 Patients should contact their ophthalmologist if the dosage of CQ or HCQ increases, if significant loss of weight occurs, or if liver or kidney disease develops. Immediate screening and ancillary testing should be considered if ocular symptoms, including nyctalopia or blurred vision, arise or if abnormalities in Amsler grid self-testing are detected.2
Because toxicity may occur in the absence of any visible funduscopic change or standard screening test, the future of effective identification of at-risk patients may lie in more sensitive but efficient, cost-effective screening. Two modalities have emerged that may assist clinicians in detecting potential toxicity in asymptomatic patients: fundus autofluorescence and multi-focal electroretinography (mfERG).1, 2, 4 Fundus autofluorescence is an imaging technology that utilizes the properties of fluorophores, specifically lipofuscin, within the retinoid cycle.5 Lipofuscin is thought to accumulate in conditions of high photoreceptor outer segment metabolism, as well as in diseased or dysfunctional RPE.5-7 Lipofuscin may be directly or indirectly toxic via oxidative stress in many retinal conditions including Stargardt’s disease, retinitis pigmentosa, age related macular degeneration, and cone dystrophies. 5, 8, 9 Increased autofluorescence is directly correlated with increased lipofuscin accumulation while the lack of normal autofluorescence indicates devitalized or atrophic RPE.
A recent study of 25 patients taking CQ or HCQ for more than one year demonstrated that FAF was effective in the early diagnosis of macular RPE disturbances.4 Abnormalities included a perifoveal ring of increased FAF in mild cases and a significant decrease in paracentral FAF in more severe cases. Fundus autofluorescence demonstrated maculopathy in all cases in which ophthalmoscopy and FA were abnormal as well as in one case in which the examination and FA were normal.
Multifocal ERG generates ERG responses arising from macular photoreceptors and likely from bipolar cells and may therefore facilitate early detection of maculopathy.1, 4, 10 Multifocal ERG allows for localization of specific areas of function within the macula and is thus gaining widespread use in detecting and confirming pathologic conditions of the macula. In HCQ toxicity, this may manifest as depressed electrophysiologic responses with a bull’s eye distribution in the mfERG tracings.4 Lyon has noted that other mfERG parameters such as ring ratios may be sensitive in detecting hydroxychloroquine induced retinal toxicity.10, 11
Patients in the higher risk group may be advised that the risk of developing retinopathy is still low but annual screening should be arranged. If toxicity is suspected based on symptoms, examination, or ancillary testing, a more in-depth assessment, including HVF testing, FA, FAF, and mfERG, should be considered. More frequent follow-up may be recommended if suspicion of toxicity remains high despite a normal evaluation or if the medication dosage is unusually high.
Other than immediate cessation of CQ and HCQ, no medical treatment exists to ameliorate retinal toxicity. There is a small chance that improvement may occur if toxicity is discovered at an early stage.2 Once toxicity is advanced, improvement is very unlikely, and progression of maculopathy may occur months after the medication has been discontinued.3, 12
Take Home Points
- Hydroxychloroquine and chloroquine have been used in the treatment of rheumatologic diseases for several decades; the overall risk of ocular toxicity is low.
- Essential screening modalities include a complete ophthalmic examination as well as assessment of the visual field.
- Typical manifestations of retinopathy include paracentral scotomas and pigmentary retinopathy, which often manifests as bull’s eye maculopathy.
- Fundus autofluorescence and multifocal ERG are promising modalities that are sensitive in the detection of early toxicity.
- Patients at higher risk for toxicity should be screened annually whereas those in the low risk category may be evaluated less frequently.
- The key to the prevention of significant and permanent vision loss is early detection of maculopathy followed by prompt discontinuation of the medication.
- Patients should be informed upon initiation of treatment with CQ or HCQ of the relatively low but real risk of vision loss associated with these medications.
Want to Subscribe to Case of the Month?
- References
- Tzekov R. Ocular toxicity due to chloroquine and hydroxychloroquine: electrophysiological and visual function correlates. Doc Ophthalmol 2005;110(1):111-20.
- Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology 2002;109(7):1377-82.
- Easterbrook M. Ocular effects and safety of antimalarial agents. Am J Med 1988;85(4A):23-9.
- Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci 2006;47(8):3531-8.
- Cideciyan AV, Aleman TS, Swider M, et al. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum Mol Genet 2004;13(5):525-34.
- Avalle LB, Wang Z, Dillon JP, Gaillard ER. Observation of A2E oxidation products in human retinal lipofuscin. Exp Eye Res 2004;78(4):895-8.
- Davies S, Elliott MH, Floor E, et al. Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic Biol Med 2001;31(2):256-65.
- Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J 2004;18(3):562-4.
- Kellner U, Kellner S, Weber BH, et al. Lipofuscin- and melanin-related fundus autofluorescence visualize different retinal pigment epithelial alterations in patients with retinitis pigmentosa. Eye (Lond) 2009;23(6):1349-59.
- Lyons JS, Severns ML. Detection of early hydroxychloroquine retinal toxicity enhanced by ring ratio analysis of multifocal electroretinography. Am J Ophthalmol 2007;143(5):801-9.
- Lyons JS, Severns ML. Using multifocal ERG ring ratios to detect and follow Plaquenil retinal toxicity: a review : Review of mfERG ring ratios in Plaquenil toxicity. Doc Ophthalmol 2009;118(1):29-36.
- Sassani JW, Brucker AJ, Cobbs W, Campbell C. Progressive chloroquine retinopathy. Ann Ophthalmol 1983;15(1):19-22.