West Coast Retina
Case of the Month
June, 2016
Presented by Ananda Kalevar, MD
A 53 year-old man presents with decreased vision in both eyes.

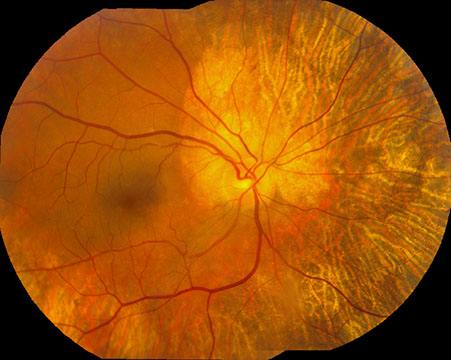
A
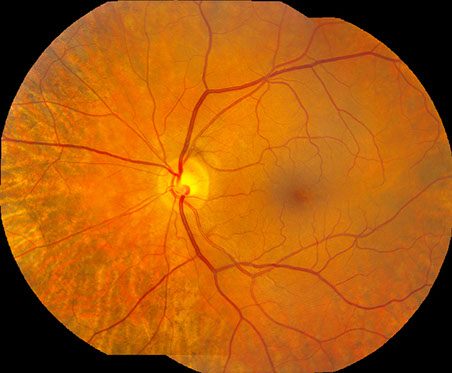
B
Figures 1A and B: Color fundus photographs of the right (A) and left (B) eyes. Note the chorioretinal atrophy in both eyes extending from the nerve, peripherally.
Case History
A 53 year-old man presents with bilateral peripheral vision loss for the past 7 years. His past medical history was significant for HIV disease, type II Diabetes Mellitus and a prior liver transplant. His medications included ondansetron, truvada (anti-retroviral combination drug consisting of emtricitabine, tenofovir), tivicay (anti-retroviral), prednisone (1 tab QD), trazodone, and nexium. His past ocular history and family history was non-contributory.
On examination, best-corrected visual acuity was 20/32 and 20/25 in the right and left eye, respectively. Intraocular pressure was normal in both eyes. The anterior segment examination was unremarkable. The posterior segment exam was remarkable for mid-peripheral retinal thinning and loss of retinal pigment epithelium with festooned borders (figure 1A-B).
Spectral domain OCT of the right eye (figure 2A and B) showed significant outer retinal loss peripapillary. SD-OCT of the left eye was unremarkable. Fundus autofluorescence of the right eye (figure 3A) showed large nummular areas of hypo-autofluorescence consistent with chorioretinal atrophy in the mid-periphery and peripapillary, with similar areas seen mid-peripherally in the left eye (figure 3B).
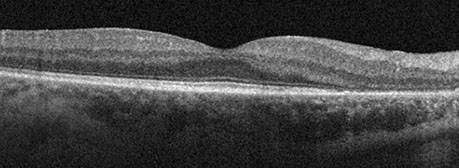
A
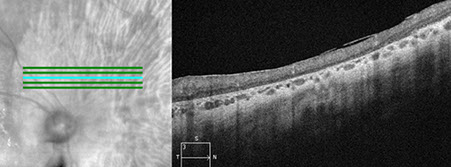
B
Figures 2A and B: Spectral Domain OCT of the right eye. Note the marked area of atrophy of the outer retina and retinal pigment epithelium in the nasal macula (A) and the peripapillary area (B)
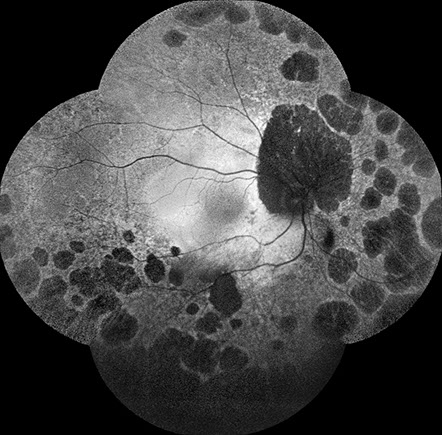
A
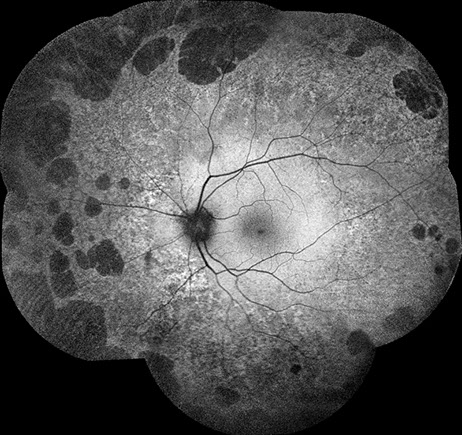
B
Figures 3A and B: Fundus autofluorescence of the right (A) and left (B) eyes. Extensive nummular shaped areas of absent and reduced oautofluorescence are seen in the periphery in both eyes.
What is your Diagnosis?
Differential Diagnosis
Mellaril toxicity, Thorazine toxicity, Gyrate atrophy, Bietti’s crystalline retinopathy, Choroideremia, Retinitis Pigmentosa, chronic progressive external ophthalmoplegia (CPEO), mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS), maternally inherited diabetes and deafness (MIDD), Rubella, Enhanced S-cone syndrome.
Additional Case History
Upon further questioning, the patient had been on Didanosine for 5 years in the past as an anti-retroviral agent. Goldmann visual field testing revealed significant constriction in both eyes, more prominently in the right (figure 4A-B). Full-field electroretinogram (ffERG) showed moderate to severe reduction in rod responses OU. There were mild to moderate reduction in cone responses OU as well. Multi-focal electroretinogram (mfERG) showed decreased responses in the peripheral macula OU. These ERG studies are consistent with a moderately advanced widespread retinal degeneration affecting rods more than cones.
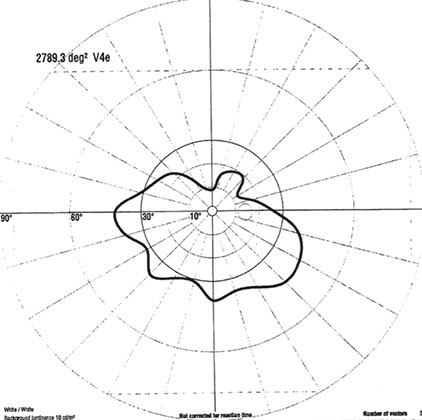
A
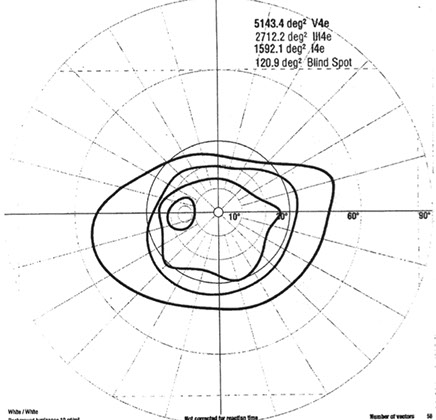
B
Figures 4A and B: Goldmann visual fields of the right (A) and left (B) eyes. Severe constriction seen in both eyes.
Discussion
Didanosine was one of the earliest nucleoside reverse transcriptase inhibitors (NRTI) used in combination treatment of HIV infection. Over the past 30 years, it has been an integral part of the highly active antiretroviral therapy (HAART). A phase I/II study of HIV infected children who were treated with didanosine revealed that 4 individuals developed didanosine related retinal toxicity.1 Subsequently, though infrequently, there have been other reported cases.2,3
The largest case series3 revealed didanosine retinal toxicity caused bilateral, symmetric, concentric mid-peripheral chorioretinal atrophy generally sparing the posterior pole. These atrophic changes could start as retinal pigment epithelium (RPE) mottling to nummular patches of atrophy. Fundus autofluorescence (FAF) imaging revealed mid-peripheral hypo-autofluorescence consistent with the areas of atrophy. Fluorescein angiography (FA) similarly showed hypo-fluorescence in the areas of atrophy. In both the FAF and FA imaging modalities, the macula was found to be spared. Due to mid-peripheral nature of the toxicity, the patient may be asymptomatic and presentation to an ophthalmologist may be delayed. Electroretinogram testing revealed severe reduction to nearly extinguished responses in photoreceptor function.
Histopathological analysis of an eye with didanosine toxicity revealed retinal pigment epithelium and chorioretinal atrophy.4 Patients had continued progression of their retinal toxicity despite cessation of the didanosine similar to what is seen in Mellaril toxicity. This has been attributed to the possible additive effect of other NRTI class drugs.
Given issues of multiple toxicities, drug interactions, inconvenience, virologic efficacy, and cost, it has been suggested that didanosine should be removed from the HAART armamentarium.5
Take Home Points
- Didanosine can cause bilateral symmetric mid-periphery chorioretinal atrophy.
- Due to this toxicity sparing the macula, presentation may be delayed.
- Despite discontinuation of Didanosine, retinal toxicity may progress.
Want to Subscribe to Case of the Month?
References
- Whitcup SM, Butler KM, Caruso R, de Smet MD, Rubin B, Husson RN, Lopez JS, Belfort R, Pizzo PA, Nussenblatt RB. Retinal toxicity in human immunodeficiency virus–infected children treated with 2′, 3′-dideoxyinosine. American journal of ophthalmology. 1992 Jan 31;113(1):1-7.
- Gabrielian A, MacCumber MM, Kukuyev A, Mitsuyasu R, Holland GN, Sarraf D. Didanosine-associated retinal toxicity in adults infected with human immunodeficiency virus. JAMA ophthalmology. 2013 Feb 1;131(2):255-9.
- Haug SJ, Wong RW, Day S, Choudhry N, Sneed S, Prasad P, Read S, Agarwal A, Davis J, Sarraf D. Didanosine Retinal Toxicity. Presented at Macula Society 2016.
- Whitcup SM, Dastgheib K, Nussenblatt RB, Walton RC, Pizzo PA, Chan CC. A clinicopathologic report of the retinal lesions associated with didanosine. Archives of Ophthalmology. 1994 Dec 1;112(12):1594-8.
- Dziuban EJ, Raizes E, Koumans EH. A farewell to didanosine: harm reduction and cost savings by eliminating use of didanosine. International journal of STD & AIDS. 2015 Oct 1;26(12):903-6.