West Coast Retina
Case of the Month
May, 2014
13-year-old boy presents with bilateral blurred vision
Presented by Sara Haug, MD, PhD

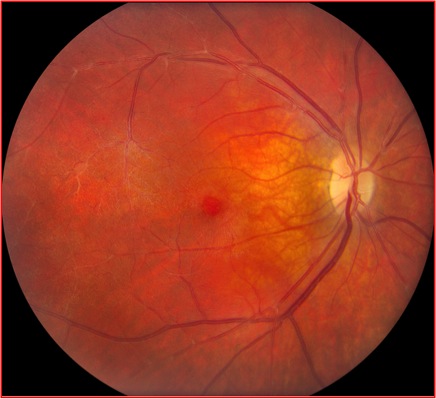
A
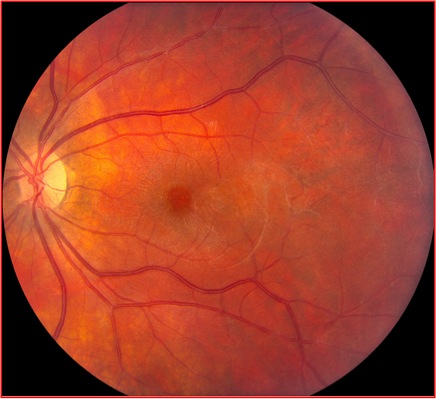
B
Figures 1A and B: Color fundus photographs of the right (A) and left (B) posterior pole. On the right (A), there are sheathed vessels observed in the posterior pole. Sheathed vessels are also seen in the left eye as well as an epiretinal membrane (B).
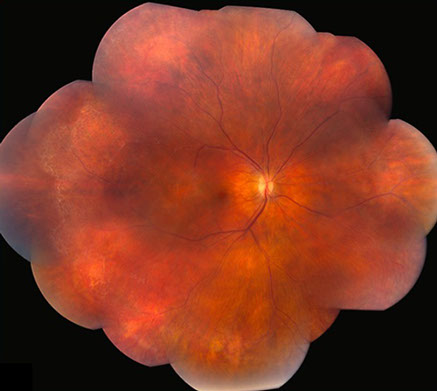
A
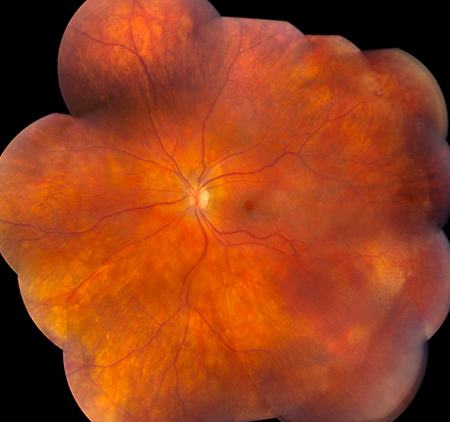
B
Figures 2A and B: Color montage photograph of the right (A) and left (B) fundus. Sheathed vessels are noted in both eyes with widespread peripheral ischemia.
A
B
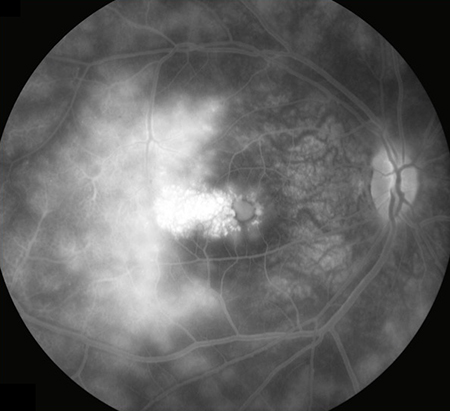
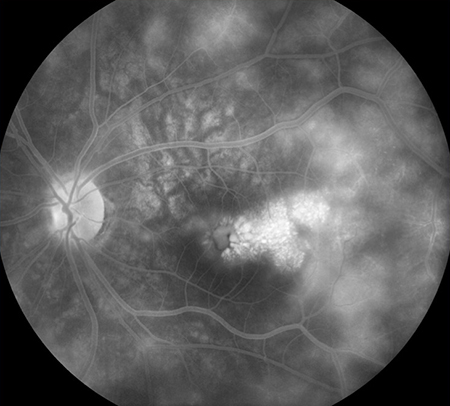
Figures 3A and B: Fluorescein angiogram of the right (A) and left (B) fundus. In both eyes, there is evidence of extensive late leakage and cystoid macular edema.
A
B
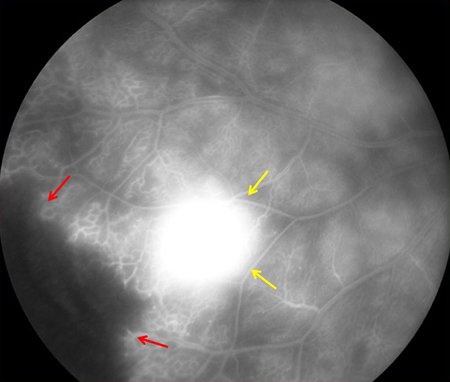
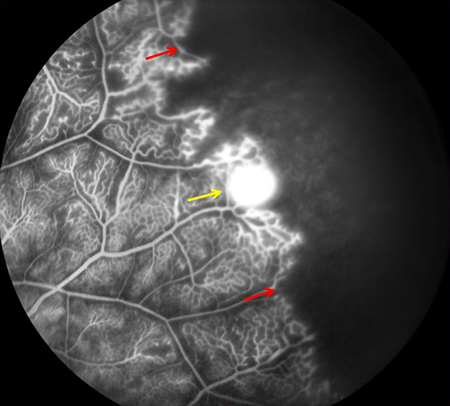
Figures 4A and B: Late phase fluorescein angiograms of the right (A) and left (B) periphery. Peripheral ischemia is noted in both eyes as well as neovascularization, shown by the yellow arrows.
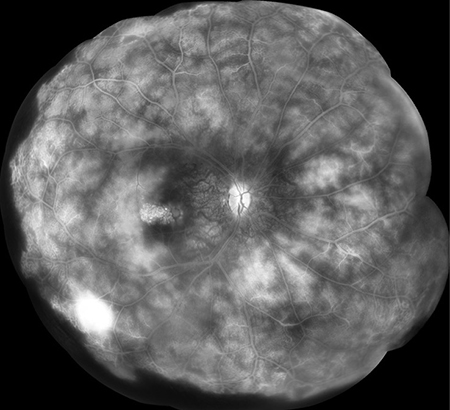
A
B
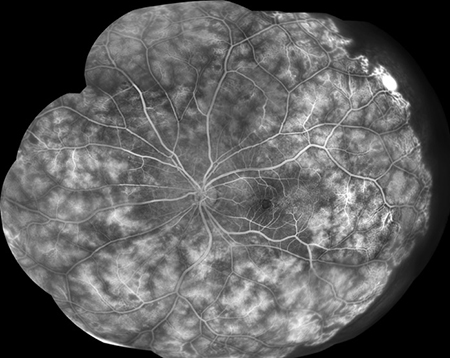
Figures 5A and B: Fluorescein angiogram montages of the right (A) and left (B) eye. Widespread telangiectasis, peripheral nonperfusion, and retinal leakage is evident in both eyes.
A
B
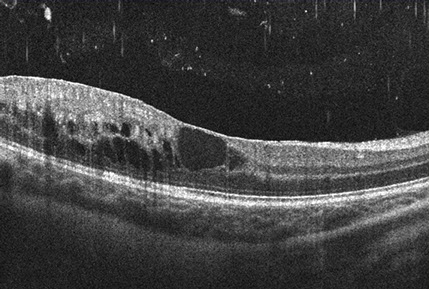
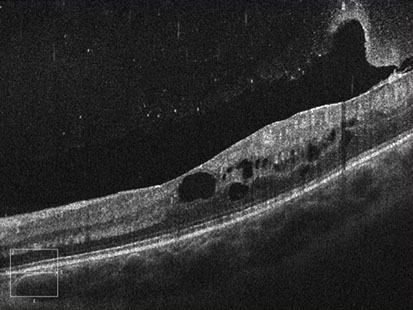
Figures 6A and B: Spectral-domain OCT images of the right (A) and left (B) eyes. Cystoid macular edema is observed in both eyes.
What is your Diagnosis?
Differential Diagnosis
The differential diagnosis for retinal leakage with telangiectasias and peripheral ischemia is broad and includes bilateral Coats disease, incontinentia pigmenti, Norrie disease, Parry-Romberg Syndrome, familial exudative vitreoretinopathy, or facioscapulohumoral dystrophy. Bone marrow disorders such as dyskeratosis congenita and Fanconi anemia also need to be considered. Other conditions such as Takayasu’s disease, Cohen syndrome, Duchenne muscular dystrophy, sickle-thalassemia, and hypereosinophilic syndromes as well as Eales disease are associated with these findings.
Additional Medical History
Additional history of our patient included sensorineural hearing loss in the right ear, first noticed at age 6. His right arm fatigued more easily than the left and is less coordinated, although he is right-handed. He has no trouble reaching over his head to throw a ball. The patient is unable to whistle and reports frequent dizzy spells. He has trouble sleeping and has leg pains most prominent at night that result in a limp the following day. Neurological exam revealed auditory extinction on the right and a symmetric face. There is bilateral scapular winging and the patient has mild difficulty walking on his toes. He struggles to keep his right foot elevated and has brisker reflexes on the right side.
With the additional medical history, incontinentia pigmenti and Norrie disease can be ruled out. Incontinentia pigmenti is extremely rare in males and involves cutaneous lesions, which our patient did not have. Norrie’s disease usually has a much more severe ophthalmic presentation and developmental delay that was not present in this young man. Our patient had no bone marrow, skin, or nail abnormalities (as in dyskeratosis congenita or Fanconi anemia), no family history of retinal disease (seen with familial exudative vitreoretinopathy), and no hemifacial atrophy (as in Parry-Romberg Syndrome).
Hypereosinophilic disease and sickle-thalassemia were ruled out by laboratory testing and the clinical picture is not consistent with Duchenne muscular dystrophy, Takayasu’s disease or Cohen syndrome. Although bilateral Coats or Eales disease could not be eliminated as possible diagnoses, the clinical picture is most consistent with facioscapulohumoral dystrophy (FSHD). Genetic testing for two types of FSHD has been negative but the clinical and ophthalmologic findings are convincing for a subcategory of FSHD patients that do not have the genetic markers identified at this time.
Discussion
FSHD is the third most common neuromuscular disorder with an incidence estimated between one in 400,000 to one in 20,000.1 The most frequent presenting symptoms are facial and shoulder girdle muscle weakness, with progression of the disease to anterior foreleg, abdominal and pelvic girdle muscles.1-3 The severity of the myopathy range from minimally detectable to disabling and is linked to the severity of the genetic changes.2,3
The inheritance pattern of the majority of FSHD cases is autosomal dominant, although a sporadic mutation is found in 10-30% of patients. FSHD patients exhibit a critical gene deletion of tandemly repeated DNA of the subtelomeric region of chromosome 4q, generating a short fragment. Genetic testing assesses the variation at this locus called the D4Z4 repeat at the subtelomeric region and the severity of disease correlates with the degree of shortening of this D4Z4 repeat.2-5 Approximately 95% of patients with FSHD will have this deletion and this is described as FSHD1. However, in a small minority of patients termed FSHD2, D4Z4 repeats are not deleted. Lemmers et al. identified an epigenetic modifier of the D4Z4 epiallele, called SMCHD1, as a causal genetic determinant of FSHD2.5 FSHD1 and FSHD2 are phenotypically indistinguishable. Our patient’s serum was sent to Dr. Lemmers’ lab in the Netherlands for further genetic testing and he was not found to have either FSHD1 or FSHD2. Upon further discussion, Dr. Lemmers reports a theoretical FSHD3, which does not currently have an identified genetic etiology but encompasses patients such as our case here with a classic clinical presentation of FSHD but negative genetic testing.6
FSHD patients, particularly those with early-inset myopathy, occasionally develop sight-threatening exudative retinopathy, which is clinically identical to Coats disease.7-10 Sensorineural hearing loss, thought to be cochlear, is often present.9 The common underlying abnormality seen in FSHD and Coat’s disease of the peripheral retinal vasculature is thought to belong to a class of developmental retinal vascular diseases called “retinal hypovascular disorders”.9,11,12 This classification includes Norrie disease, familial exudative vitreoretinopathy, and osteoporosis pseudoglioma syndrome. These developmental retinal hypovasculopathies also share common pathogenic pathways caused by mutation of one of the components of the Wnt signaling cascade present on retinal endothelium.9,11,12 This signaling cascade is also fundamental to skeletal myogenesis and muscle regeneration as well as the cochlear striae vascularis.9,11,12
Take Home Points
- The differential diagnosis for bilateral retinal telangiectasis and peripheral ischemia is broad and requires an extensive medical and neurological evaluation.
- The Wnt signaling cascade present on the retinal vascular endothelium underlies many of these retinal hypovascular disorders and can also affect myogenesis and muscle regeneration as well as chochlear function.
Want to Subscribe to Case of the Month?
References
- Gonzalez-Navarro FF, Belanche-Munoz LA, Silva-Colon KA. Effective classification and gene expression profiling for the Facioscapulohumeral Muscular Dystrophy. PLoS One 2013;8:e82071.
- Lemmers RJLF, O’Shea S, Padberg GW, et al. Best practice guidelines on genetic diagnostics of facioscapulohumoral muscular dystrophy: Workshop 9th June 2010, LUMC, Leiden, The Netherlands. Neuromuscul Disord 2012;22:463-70.
- Sacconi S, Camano P, de Greef JC, et al. Patients with a phenotype consistent with facioscapulohumeral muscular dystrophy display genetic and epigenetic heterogeneity. J Med Genet 2012;49:41-6.
- Wijmenga C, Frants RR, Brouwer OF, et al. Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet 1990;336:651-3.
- Lemmers RJLF, Tawil R, Petek LM, et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral dystrophy type 2. Nat Genet 2012;44:1370-4.
- Dr. Lemmers, personal communication. March, 2014
- Wulff JD, Lin JT, Kepes JJ. Inflammatory facioscapulohumeral muscular dystrophy and Coats syndrome. Ann Neurol 1982;12:398-401.
- Pauleikhoff D, Bornfield N, Bird AC, et al. Severe visual loss associated with retinal telangiectasis and facioscapulohumeral muscular dystrophy. Graefe’s Arch Clin Exp Ophthalmol 1992;230:362-5.
- Fitzsimons RB. Retinal vascular disease and the pathogenesis of facioscapulohumeral muscular dystrophy. A signaling message from Wnt? Neuromuscul Disord 2011;21:263-71.
- Vance SK, Wald KJ, Sherman J, et al. Subclinical facioscapulohumeral muscular dstrophy masquerading as bilateral Coats disease in a woman. Arch Ophthalmol 2011;129:807-9.
- Clevers H. Eyeing up new Wnt pathway players. Cell 2009;139:227-9.
- Block GJ, Narayana D, Amell AM, et al. Wnt/beta-catenin signaling suppresses DUX4 expression and prevents apoptosis of FSHD muscle cells. Hum Mol Genet 2013;22:4461-72.