West Coast Retina
Case of the Month
September, 2010
Presented by Sandeep Randhawa, MD
A 78-year-old man with periodic decrease in vision in his right eye.

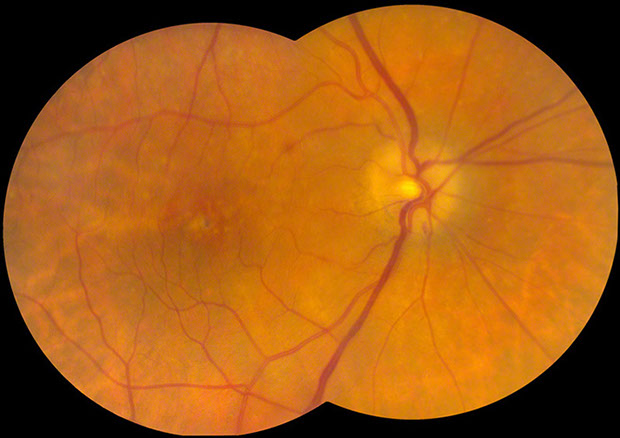
Figure1: Color fundus photograph of the right eye. Note the mild optic disc edema with splinter disc hemorrhage. A small retinal hemorrhage is present in the papillomacular bundle.
Case History
A 78-year-old man presented with periodic decreased vision in his right eye for two days. His past medical history was significant for colitis. The only recent change to his health was a ‘viral illness’ which he had developed 3 days prior to presentation.
On examination, his visual acuity was 20/80 in the right eye, which was decreased from a baseline of 20/40. His visual acuity was 20/40 in the left eye. The intraocular pressure and confrontation visual field were normal in both eyes. Slit lamp examination of the anterior segment was notable only for mild nuclear sclerotic cataract.
A dilated fundus examination of the right eye revealed a slightly blurred optic disc margin with an inferior disc hemorrhage, a small retinal hemorrhage in the papillomacular bundle, pigment mottling with drusen in the macula, normal retinal vasculature, and peripheral retina (Fig 1). Dilated fundus examination of the left eye revealed a normal optic disc, macula, vasculature, and a few peripheral drusen.
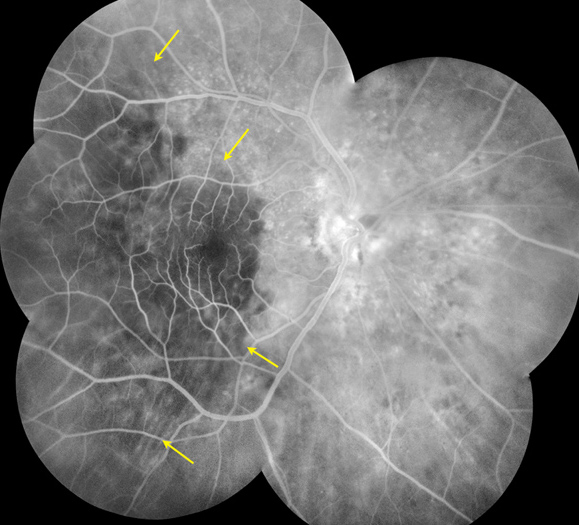
Figure 2: Fluorescein angiography of the right eye showing a wedge-shaped choroidal filling defect extending from the macula temporally (yellow arrows)
What is your Diagnosis?
Differential Diagnosis
The history and examination is significant for decreased vision in a 78-year-old man in the setting of disc edema, macular pigmentary changes, and a wedge-shaped area of choroidal hypoperfusion on fluorescein angiography. The differential diagnosis of wedge-shaped choroidal hypoperfusion defects is extensive.
Triangular or wedge-shaped choroidal lesions have classically been described with their apex pointing posteriorly and the base anteriorly. They are believed to represent evidence of occlusion of the long or short posterior ciliary arteries (PCA) or their branches.1, 2
Amalric3 divided the clinical situations in which choroidal triangles occurred into three groups: (1) generalized or systemic vascular processes; (2) localized circulatory obliterations; and (3) choroidal arteriosclerosis. Clinical entities such as malignant hypertension, chronic renal failure, toxemia of pregnancy, collagen vascular diseases (e.g., scleroderma, systemic lupus erythematosus, Raynaud's phenomenon, polyarteritis nodosa), sickle cell disease, internal carotid artery obstruction and vasculitis (e.g., temporal arteritis, Wegener’s granulomatosis, Churg-Strauss syndrome and microscopic polyangitis) are included under generalized or systemic vascular processes. Other causes include hemorrhage leading to hemorrhagic shock, and hematologic diseases such as polycythemia vera, disseminated intravascular coagulopathy, thrombotic thrombocytopenic purpura, and cryoglobulinemia. Localized circulatory obliterations refer to thromboembolic events and to ischemia resulting from entities such as trauma, infection, or degenerative disease states. Choroidal arteriosclerosis has been related to changes brought on by diabetes and aging. Choroidal ischemia has also been reported following krypton laser photocoagulation and photodynamic therapy.4, 5
Clinical Course
The patient’s blood pressure was normal. The presence of optic disc edema with concomitant choroidal hypoperfusion raised suspicion for giant cell arteritis (GCA). The erythrocyte sedimentation rate was obtained elevated to 82mm/hr. A subsequent temporal artery biopsy confirmed GCA.
He was started on oral prednisone 60mg/day. On follow-up examination, his vision had improved back to his baseline of 20/30 in his right eye and fundus examination revealed a wedge-shaped geographic patch of pigment epithelial atrophy pointed towards the disc (‘Amalric sign’), corresponding to the area of previous choroidal infarction.6
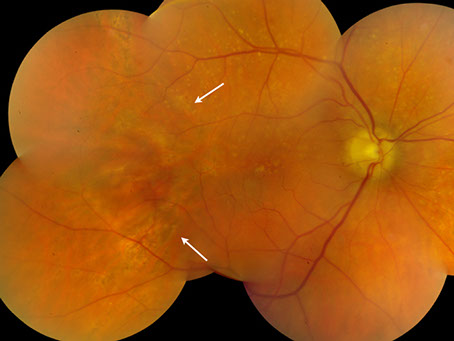
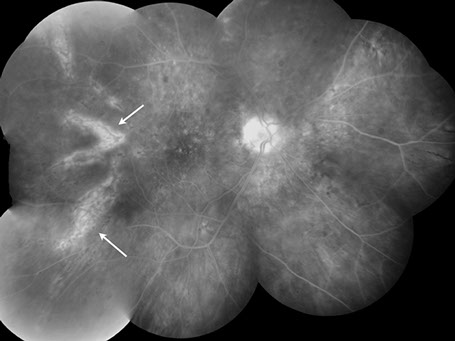
Figures 3: Color fundus photograph and fluorescein angiogram of the right eye on follow-up showing a wedge-shaped geographic patch of pigment epithelial atrophy and chorioretinal pigmentary changes in the area of previous choroidal infarction (white arrows).
Discussion
GCA is an immune-mediated granulomatous vasculitis, affecting medium- to large-sized arteries, in individuals over the age of 50 years.
‘Occult’ GCA may manifest through ocular symptoms and signs alone, preceding the systemic manifestations often by days and weeks or without systemic features. In Hayreh’s series 21.2% of patients with visual loss due to GCA had occult GCA, i.e. no systemic symptoms whatsoever, with a positive temporal artery biopsy and visual loss.7 In the absence of systemic features of GCA it becomes difficult to make a diagnosis of arteritic anterior ischemic optic neuropathy (AION), as was the case in our patient. It is critical to recognize the true ocular signs and symptoms of GCA including diplopia, and transient monocular vision loss because if left untreated, it can lead to irrevocable vision loss. Permanent visual loss in GCA usually results from arteritic AION, and less frequently from posterior ischemic optic neuropathy, retinal artery occlusion, choroidal ischemia, optic chiasm or postchiasmal pathway ischemia. Cortical blindness related to vertebrobasilar artery involvement is a rare complication of GCA.8 Arteritic AION, seen in giant-cell arteritis, is due to thrombotic occlusion of any one of the PCAs. Occlusion of the PCA may also produce extensive regional triangular wedge-shaped choroidal infarcts and helps differentiate arteritic from non-arteritic AION.9 This is very well demonstrated by fundus fluorescein angiography, performed soon after the visual loss. As time passes, choroidal filling defects tend to resolve slowly by collateral circulation. Thus, fluorescein angiography during the early stages constitutes a critical diagnostic test for arteritic AION and GCA.
Take Home Points
- Choroidal ischemia is a common but infrequently recognized complication of giant cell arteritis.
- Identification of delayed choroidal perfusion on fluorescein angiography may lead to more rapid diagnosis and treatment of giant cell arteritis, and may assist in making the diagnosis where prodromal symptoms are absent, where inflammatory markers are normal, or where it is difficult to promptly obtain temporal artery biopsy.
Want to Subscribe to Case of the Month?
References
- Hayreh SS. In vivo choroidal circulation and its watershed zones. Eye 1990;4:273-89.
- Amalric P. Choroidal vascular ischaemia. Eye 1991;5:519-27.
- Amalric P. Acute bird-shot chorioretinal ischemia. Bull Soc Fr Ophtalmol 1985;85:949-52.
- Johnson RN, Schatz, H. Delayed Choroidal Vascular Filling Following Krypton Laser Photocoagulation. Am J Ophthalmol 1985;99:154-8.
- Isola V, Pece A, Parodi MB. Choroidal ischemia after photodynamic therapy with verteporfin for choroidal neovascularization. Am J Ophthalmol 2006;142:680-3.
- Amalric P. Triangular shaped choroidal alterations. Mod Probl Ophthalmol 1971;9:68-77.
- Hayreh SS, Podhajsky PA, Zimmerman B. Occult giant cell arteritis" ocular manifestations. Am J Ophthalmol 1998;125:521-6.
- Miller NR. Visual manifestations of temporal arteritis. Rheum Dis Clin North Am 2001;27:781-97.
- Hayreh SS. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci 2004;45: 749-57; 748.