April, 2020
Presented by Caleb Ng, MD


Presented by Caleb Ng, MD

A 61-year-old Caucasian male patient is referred for evaluation of decreased vision in the right eye.
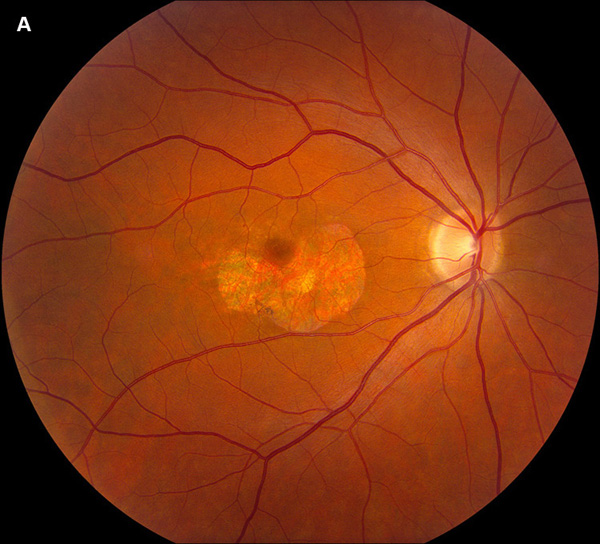
Figure 1A: Color photograph of the right eye. Note perifoveal area of geographic atrophy.
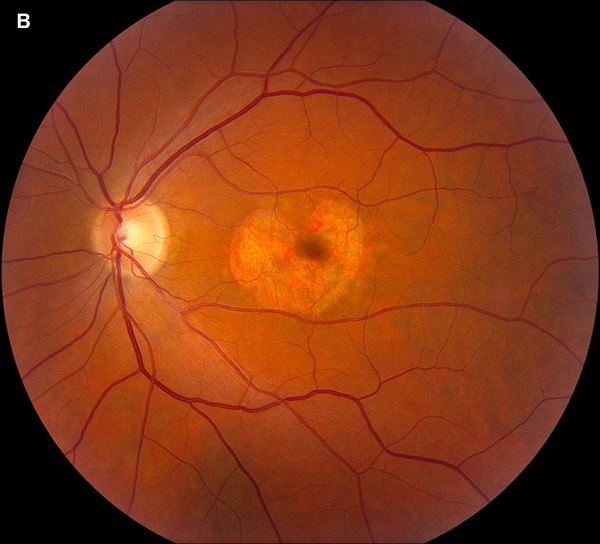
Figure 1B: Color photograph of the left eye. Note perifoveal area of geographic atrophy that is more extensive than present in the right eye.
Patient’s best corrected visual acuity was 20/32 and 20/25-2 in right and left eyes, respectively. Intraocular pressure was 10 in both eyes. Anterior segment exam was unremarkable. Posterior segment exam was remarkable for 180 degrees of inferior parafoveal atrophy in the right eye, and near circumferential parafoveal atrophy in the left eye. (Figure 1) Spectral domain ocular coherence tomography of the macula revealed outer retinal atrophy that spares the fovea. (Figure 2) At the area of retinal atrophy, fundus autofluorescence showed hypoautofluorescence with a rim of hyperautofluorescence (Figure 3), while fluorescein angiography revealed early hyperfluorescence consistent with window defect (Figure 4).
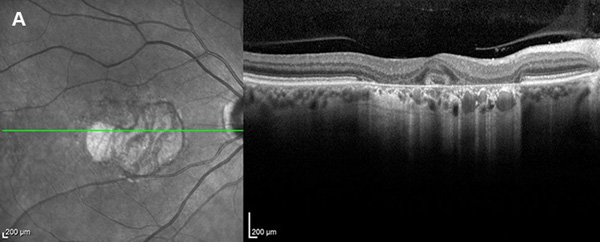
Figure 2A: OCT of the right eye. Note perifoveal area of atrophy of the outer retinal layers including the RPE (seen with increased transmission to choroid.
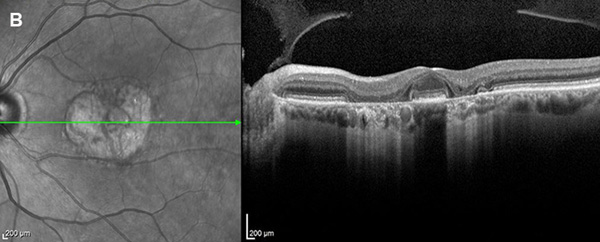
Figure 2B: OCT of the left eye. Note perifoveal area of atrophy of the outer retinal layers including the RPE (seen with increased transmission to choroid.
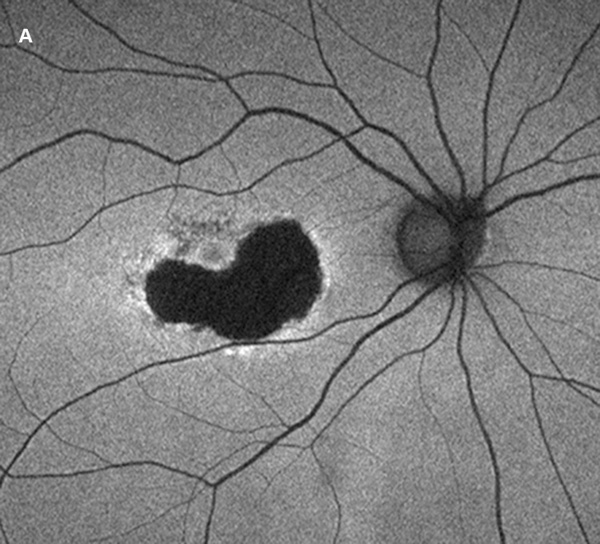
Figure 3A: Fundus Autofluorescence of the right eye. There is predominantly marked reduced autofluorescence (AF) but a ring of increased AF at the margin.
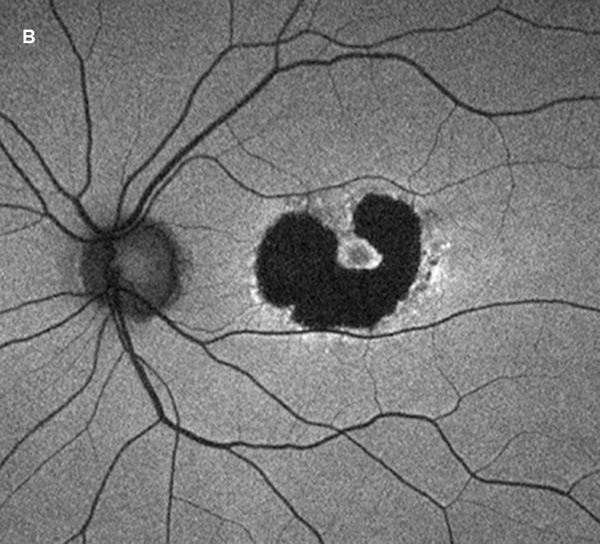
Figure 3B: Fundus Autofluorescence of the left eye. There is a larger area of reduced autofluorescence (AF) surrounding the fovea (as compared with right eye but a ring of increased AF at the margin.
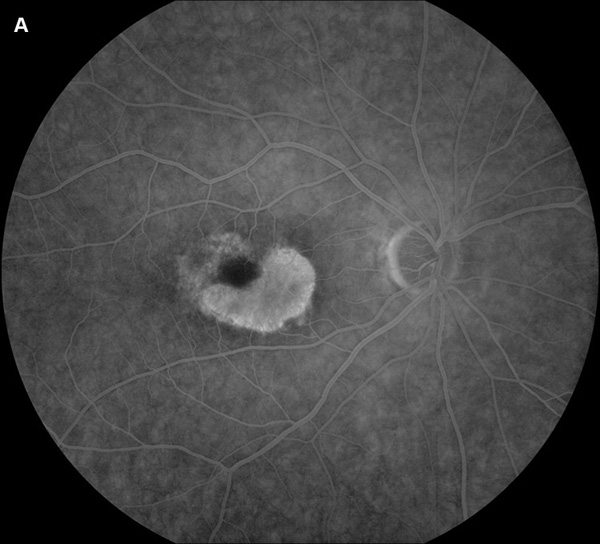
Figure 4A: Fluorescein angiogram of the right eye. Note perifoveal area of increased fluorescence due to the RPE atrophy creating a window defect.
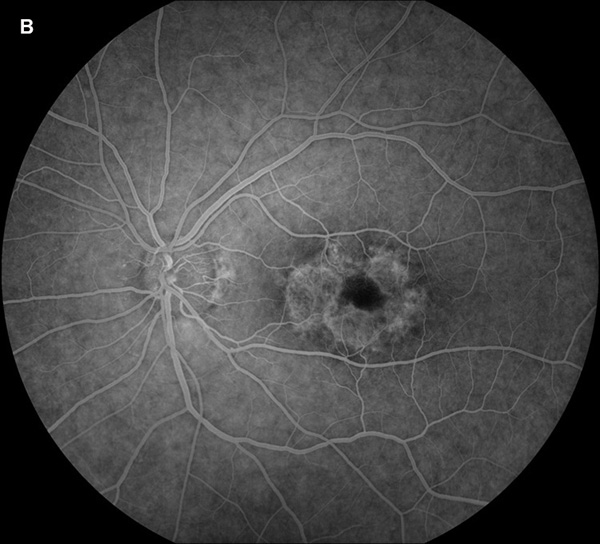
Figure 4B: Fluorescein angiogram of the left eye. Note perifoveal area of increased fluorescence due to the RPE atrophy creating a window defect.
Our patient has no previous medical or surgical history, takes no medications, is a non-smoker, and only drinks alcohol socially. He has no known family history of vision loss. Photopic and scotopic full field ERG and EOG results were grossly normal, while multifocal ERG showed depressed traces centrally. (Figure 5) Genetic testing was done and returned positive for a heterozygous CRX mutation (c.268C-T, p.Arg90Trp). We diagnosed our patient with adult-onset macular dystrophy that simulates benign concentric annular macular dystrophy (BCAMD).
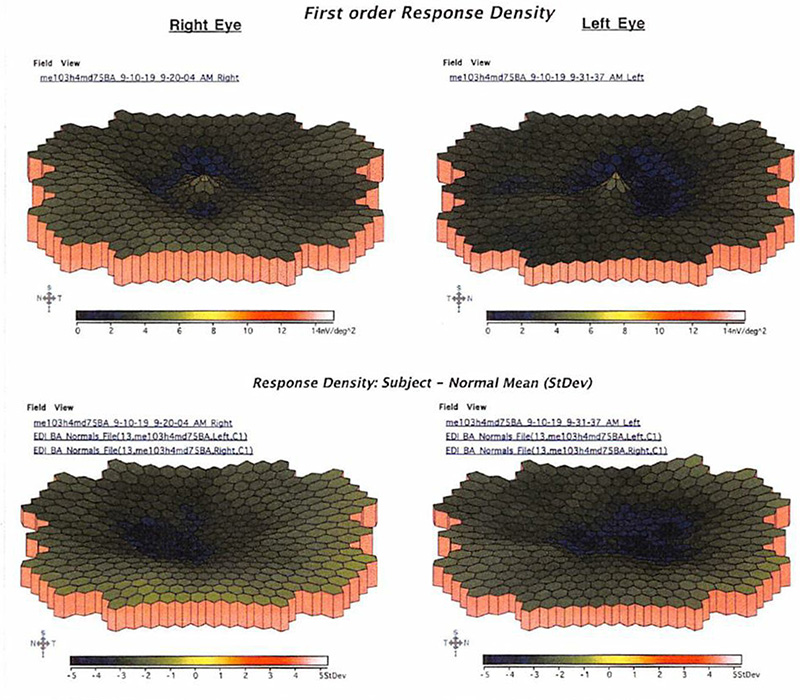
Figure 5: Multifocal ERG. Note the reduction in perifoveal amplitudes
In 1974, Deutman described 4 patients within 3 generations of the same family with bull’s eye maculopathy in the absence of exposure to Plaquenil or symptoms of cone dystrophy, and coined the autosomal dominant dystrophy as benign concentric annular macular dystrophy.1 Attempts to locate a causative gene for BCAMD resulted in the discovery of a mutation in the interphotoreceptor matrix proteoglycan 1 (IMPG1) gene.2 IMPG1 encodes for chondroitin sulfate proteoglycan core proteins that are thought to be produced by photoreceptors and retinal pigment epithelial (RPE) and Müller cells to keep the retina and RPE attached.3
Our patient’s history and exam suggest a diagnosis of BCAMD, but a three-generation family tree for our patient revealed no other individual with similar symptoms or known exam findings. Genetic analysis for our patient yielded a heterozygous CRX mutation (c.268C-T, p.Arg90Trp). Cone-Rod Homeobox (CRX) gene is located on chromosome 19; it is a photoreceptor-specific homeodomain transcription factor that regulates photoreceptor associated genes, such as rhodopsin.4,5 CRX mutations have been found in association with cone-rod dystrophy, cone dystrophy, Leber congenital amaurosis, and retinitis pigmentosa, and more recently various novel forms of adult-onset macular dystrophy.6-8 Interestingly, family members with identical CRX mutations have been observed to have phenotypic heterogeneity.8
Adult-onset macular dystrophy manifests with a broad spectrum of phenotypes and has been associated with multiple different mutations within the CRX gene. One series of 6 patients had presenting age between 35 to 50, and visual acuities ranging from 20/32 to 20/630 (Snellen).8 In our review, we found one other report of Adult onset macular dystrophy due to CRX mutation with findings very similar to BCAMD.7