May, 2021
Presented by Caleb Ng, MD


Presented by Caleb Ng, MD

A 46-year-old Hispanic woman presented with chief complaint of decreased vision and sustained temporal photopsias in her right eye.
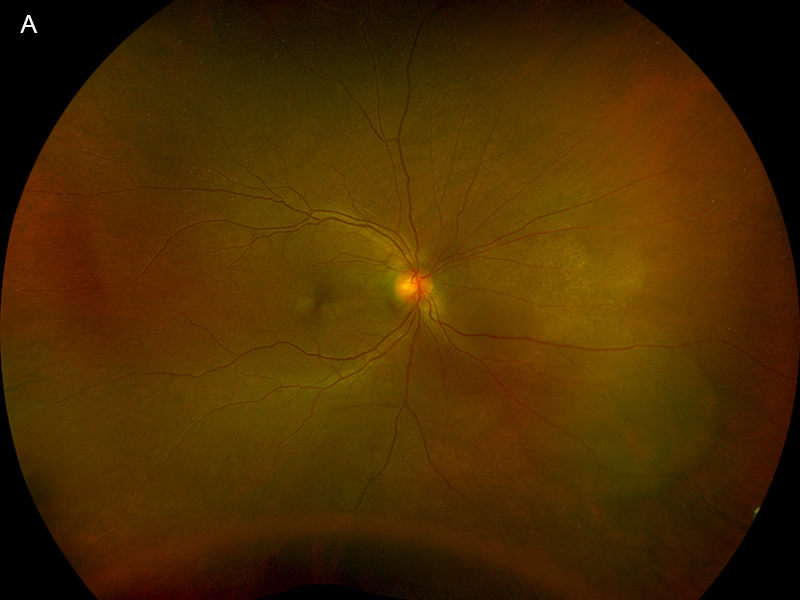
Figure 1A: Wide-field color photo of the right eye. Note the yellowish area in the nasal retina.
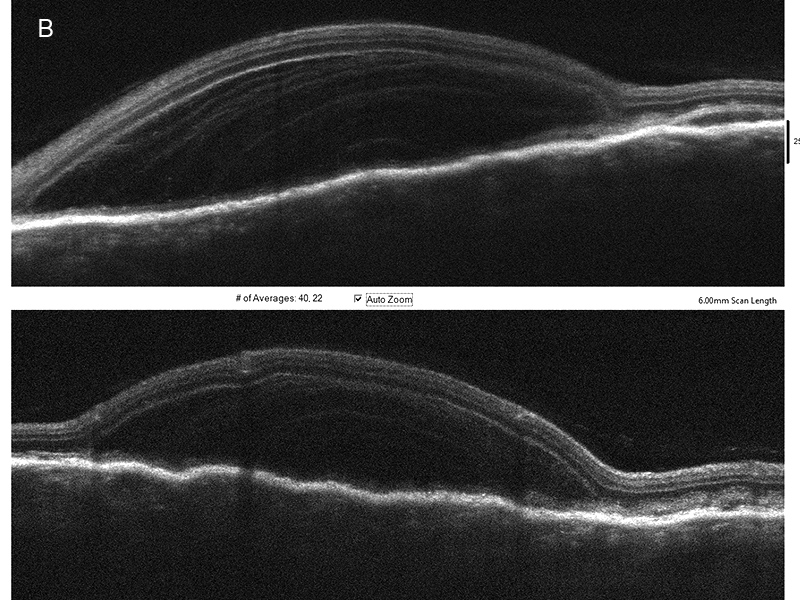
Figure 1B: SD-OCT scan through the nasal lesion. A bacillary layer detachment is noted with organized exudate in the layers.
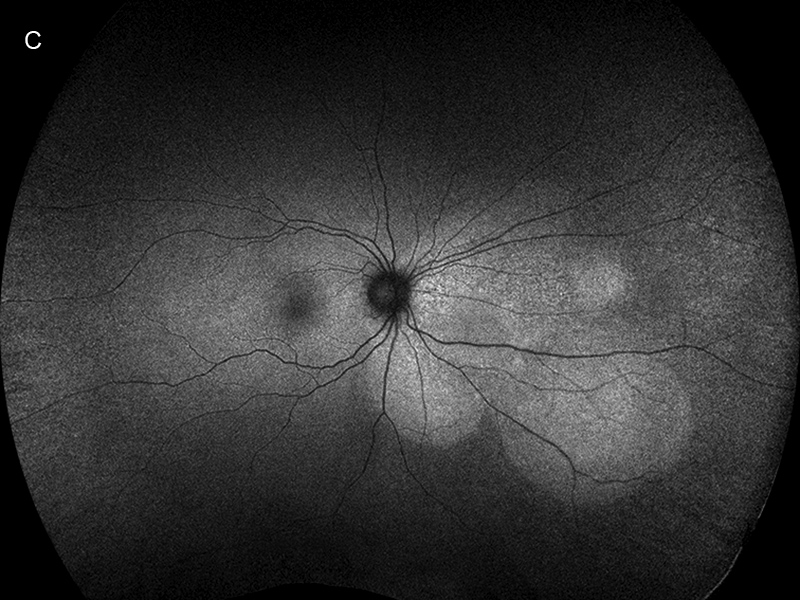
Figure 1C: Wide-field autofluorescent image of the right eye. Note the round smoothly contoured hyperfluorescent areas.
On examination, her best corrected visual acuity was 20/50 in the right eye and 20/32 in the left, and intraocular pressures were normal. Both anterior segments and her left posterior segment were unremarkable. A creamy lesion with underlying choroidal thickening and surrounding subretinal fluid was identified nasal to the optic nerve in the right eye (Figure 1A). Optical coherence tomography (OCT) through the lesion revealed a bacillary layer detachment with organized exudate in layers, and underlying chorioretinal folds (Figure 1B). The affected areas were hyperautofluorescent with smooth borders (Figure 1C). Her past medical history consisted of iron deficiency anemia and metastatic clear cell renal cell carcinoma in remission, after ipilimumab and nivolumab induction therapy, nephrectomy, and then ongoing nivolumab infusions every two weeks for two years.
Differential Diagnosis
Patient Course and Diagnosis
We relayed our concern for possible cancer recurrence with new choroidal metastasis to the patient’s oncologist, who undertook pan-body imaging and held nivolumab until the work up for metastases could be completed. Prompt MRI of the brain and orbit, and CT of the chest, abdomen, and pelvis, detected no evidence of metastases. No further interventions were initiated.Two-and-a-half weeks later, follow-up evaluation showed that the patient’s posterior segment abnormalities had largely resolved, leaving behind only an area of retinal pigment epithelium (RPE) disruption (Figure 2A). Repeat OCT through the lesion showed mildly thickened choroid with chorioretinal folds without intraretinal fluid (Figure 2B). The affected area showed interval increase in autofluorescence (Figure 2C) and transmission hyperfluorescence on fluorescein angiography (FA; Figure 2D). At this point, the patient’s oncologist resumed her nivolumab treatments, which had been interrupted for one dose.
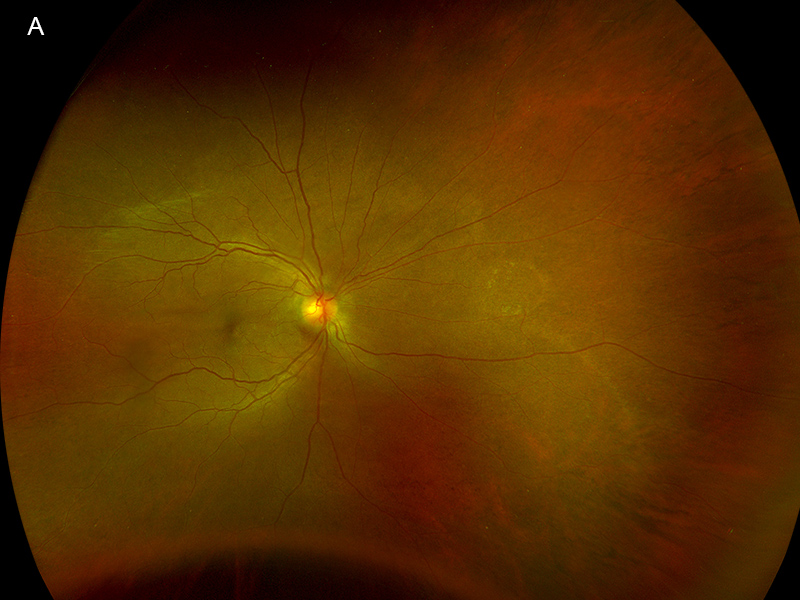
Figure 2A:Wide-field color photograph of the right eye. Note that the area of serous detachment has resolved.
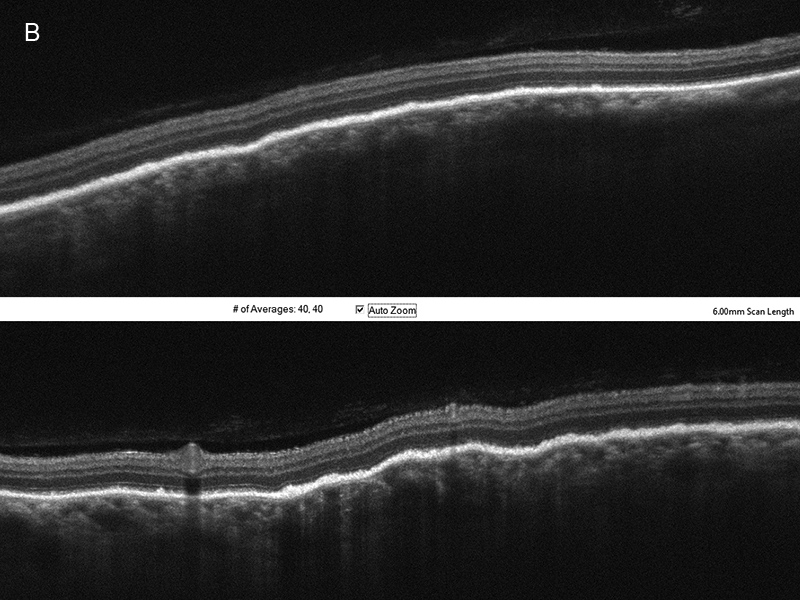
Figure 2B: SD-OCT of the nasal retina of the right eye. Note that the serous detachment has resolved. Some choroidal thickening is noted as well as choroidal folds.
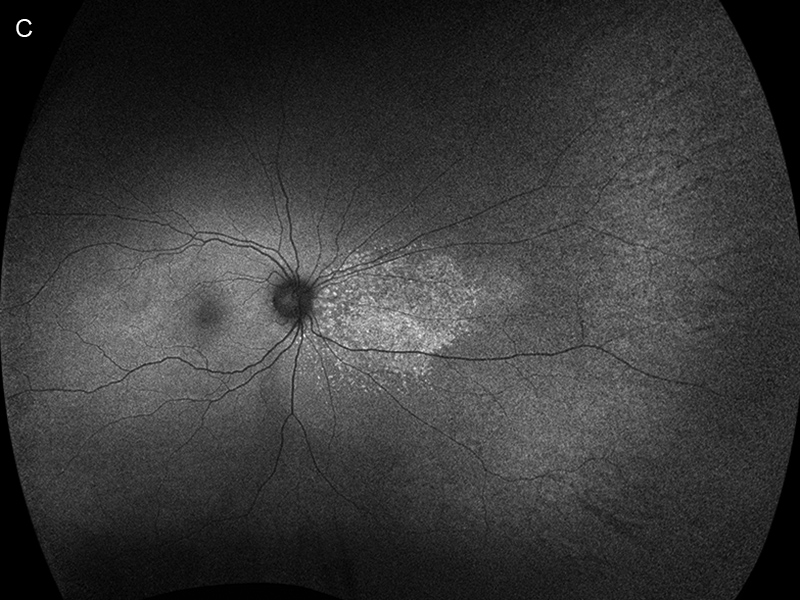
Figure 2C: Wide-field autofluorescent image of the right eye. Note the area of increased autofluorescence nasally.
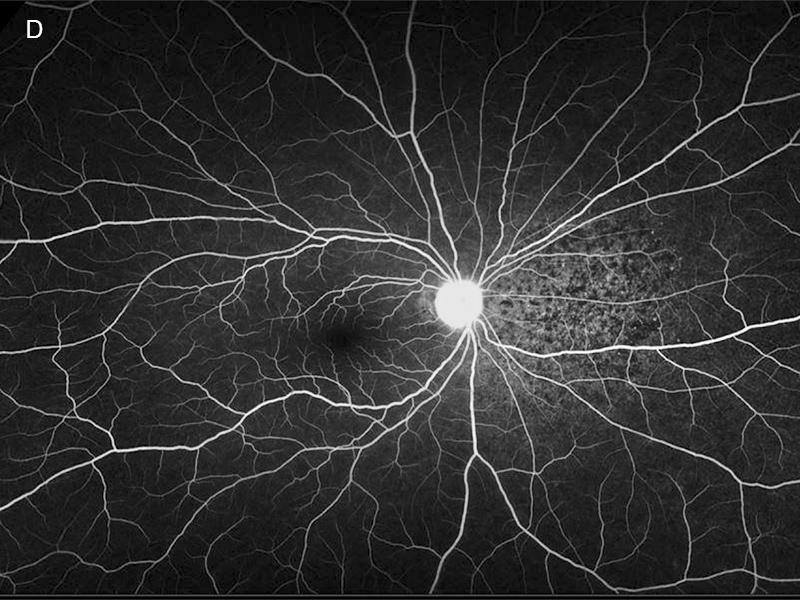
Figure 2D: Wide-field fluorescein angiogram of the right eye. The nasal retina shows a mottled area of hyperfluorescence in the area of the prior serous detachment.
Two months later she returned with decreased vision, now measuring 20/200 on the right and 20/40 on the left. Examination of the right eye showed a new creamy colored area temporally with subretinal fluid (Figure 3A). Additionally, choroidal folds were now visible clinically and on OCT in both eyes (Figure 3A,B and D). An OCT through the new lesion in the right eye showed another bacillary layer detachment (Figure 3C). Increased autofluorescence was detected in areas corresponding to the creamy lesion and surrounding subretinal fluid (Figure 3E). The nasal area of increased mottled autofluorescence had enlarged. No abnormal autofluorescence was noted in the left eye. Fluorescein angiography showed pooling of dye at the location of the bacillary layer detachment with surrounding mottled hypofluorescence and punctate foci of hyperfluorescence (Figure 3F). Indocyanine green angiography revealed hypofluorescence corresponding to the area of the bacillary layer detachment on the right (Figure 3G), but was unremarkable in the left eye. B-scan ultrasonography identified no choroidal mass or sub-Tenons fluid, or a ‘posterior T-sign’, but found diffuse bilateral choroidal thickening, with a serous retinal detachment on the right (Figure 3H).
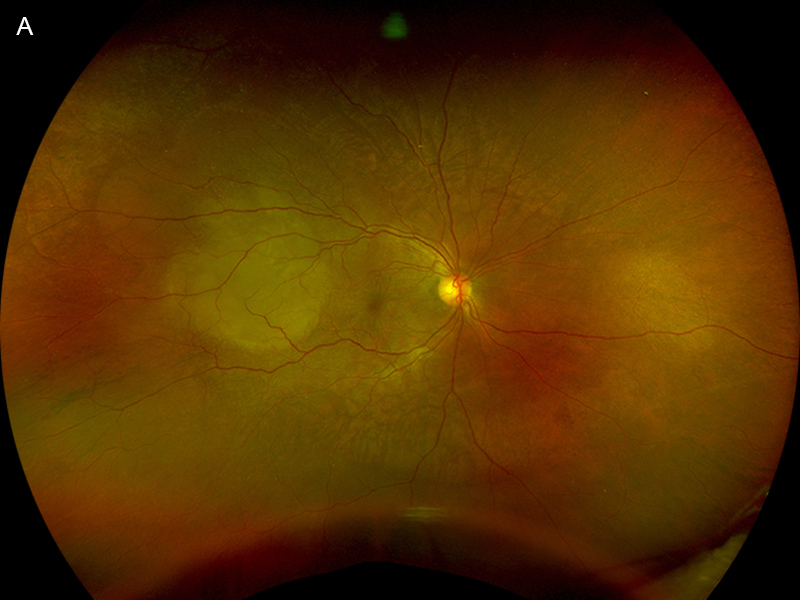
Figure 3A:Wide-field color photograph of the right eye. Note that the creamy area of new serous detachment temporally.
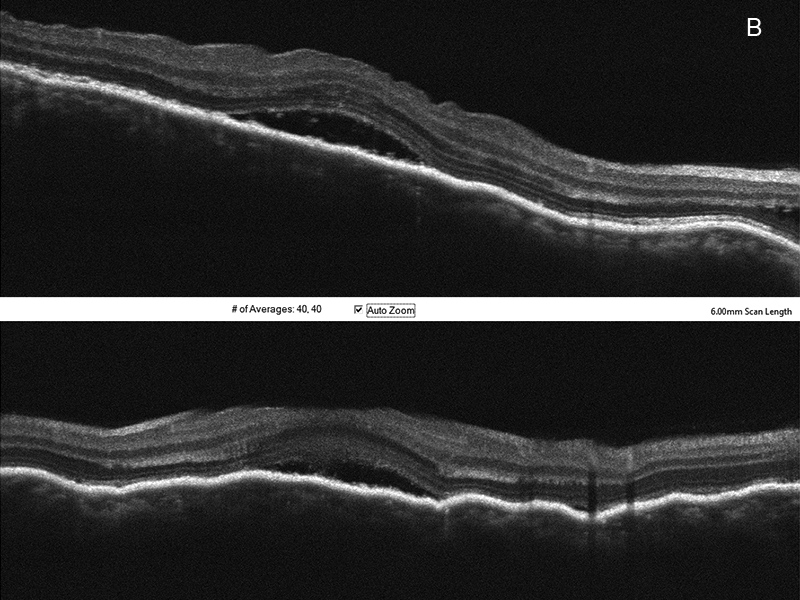
Figure 3B:SD-OCT of the right macula. Horizontal and vertical scans show serous fluid, and choroidal folds.
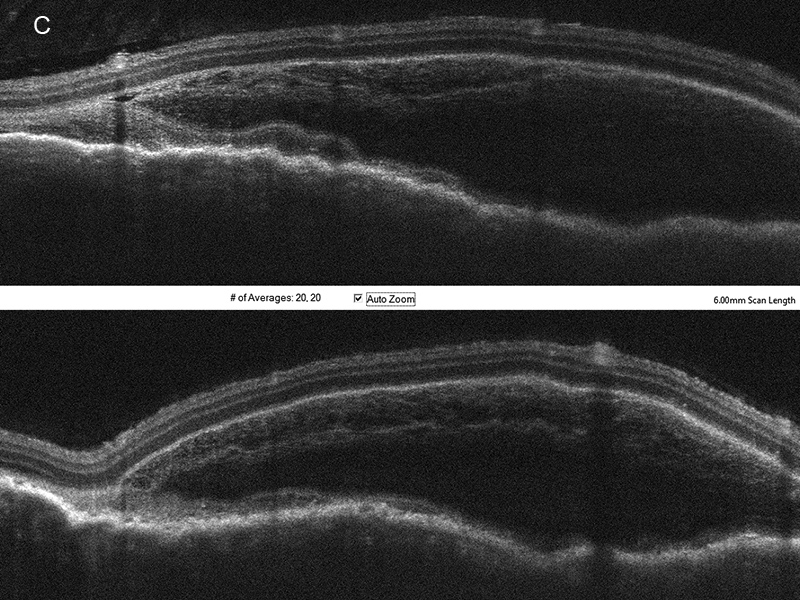
Figure 3C:Horizontal and Vertical scans through the area of serous fluid temporally in the right eye reveals a bacillary layer detachment.
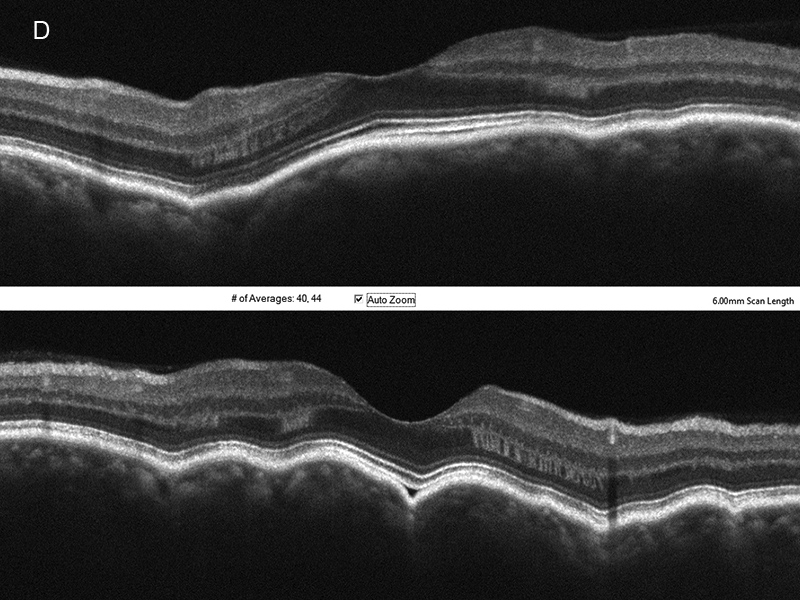
Figure 3D:Horizontal and vertical SD-OCT scans through the left macula. Choroidal folds are present.
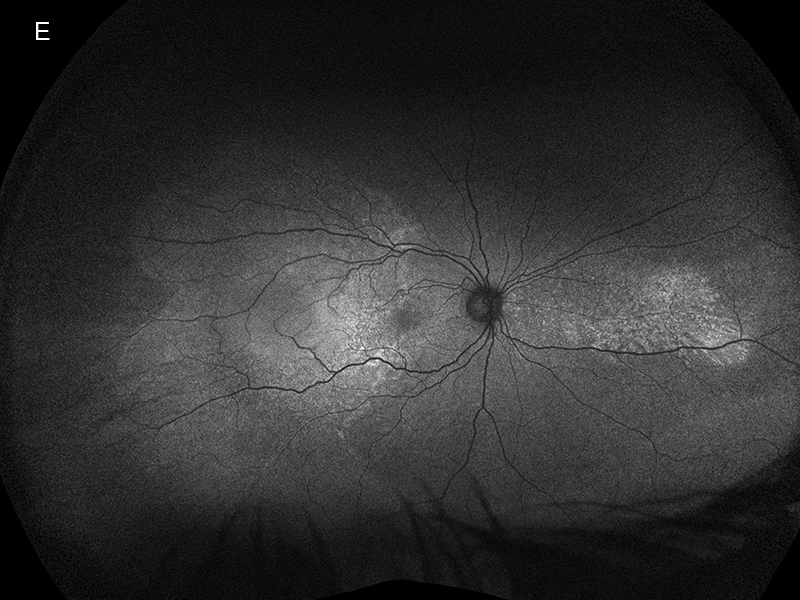
Figure 3E:Wide-field autofluorescence scan of the right eye. There is a new area of autohyperfluorescence temporally, and the nasal area has enlarged.
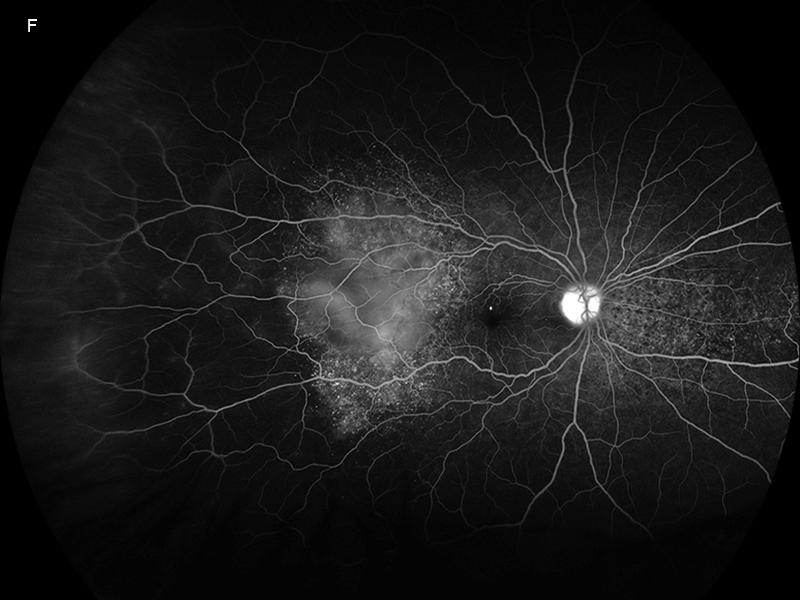
Figure 3F:Wide-field fluorescein angiogram of the right eye. Pooling is noted temporally in the area of serous detachment.
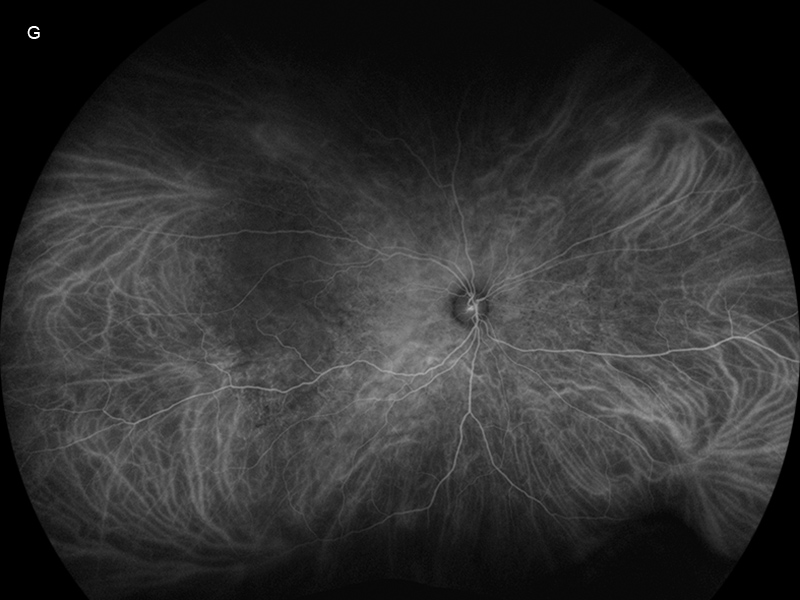
Figure 3G:Wide-field indocyanine green angiogram of the right eye. Hypofluorescence is present temporally in the area of serous detachment.
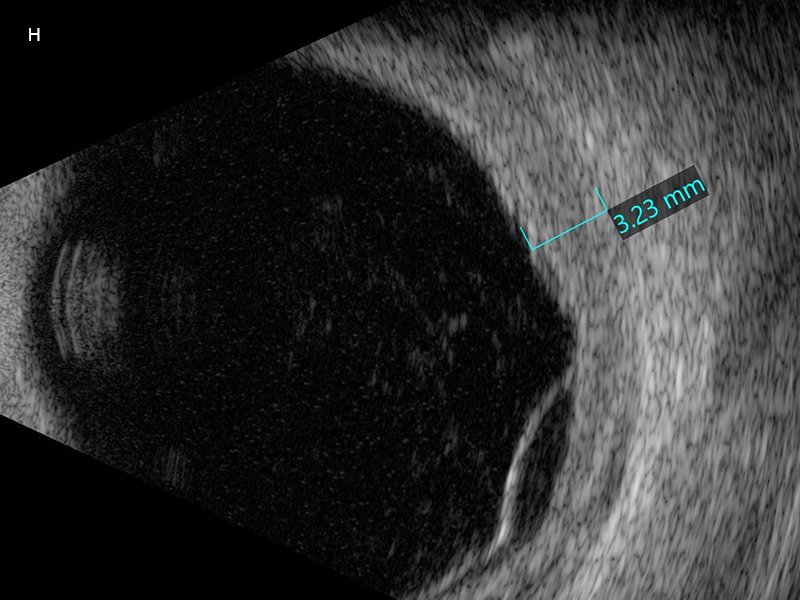
Figure 3H:B-scan ultrasound of the right eye showing choroidal thickening and retinal detachment.
Suspecting the possibility of nivolumab toxicity, we discontinued the patient’s treatment and observed her for three weeks without clinical improvement. She was then treated with 60 mg per day of oral prednisone, which led to the resolution of her choroidal thickening, serous retinal detachment, and bacillary layer detachment (Figure 4). Seven months after initial presentation, and seven weeks post steroid therapy, her vision was 20/40 on the right, and 20/32 on the left and examination of her posterior segment showed diffuse choroidal hypopigmentation with sunset glow fundus bilaterally.
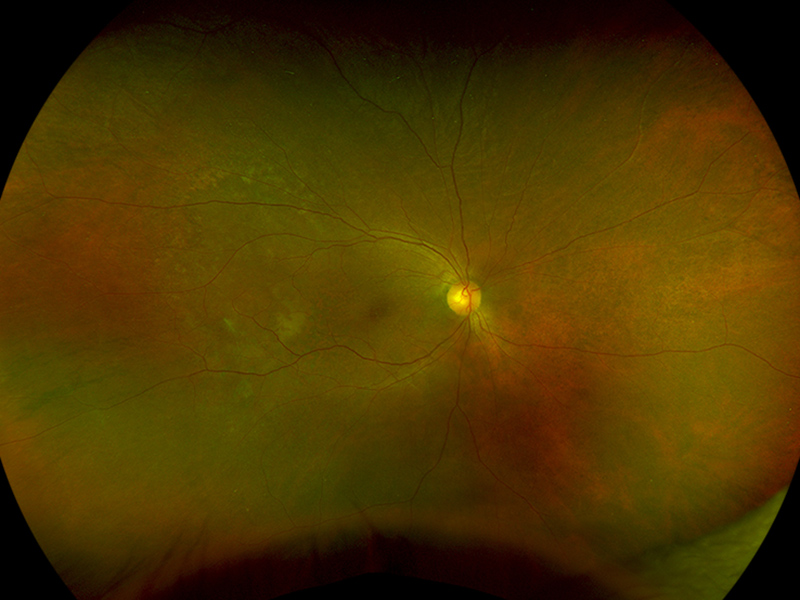
Figure 4:Wide-field color photograph of the right eye following a course of oral prednisone. The area of serous detachment has resolved.
Discussion
Nivolumab (Opdivo, Bristol-Myers Squibb Company, New York, NY), a monoclonal antibody against programmed cell death protein 1, is an anti-cancer agent bioengineered to prevent tumor cells from evading host immunity through activation of immune checkpoints. Ocular immune-related adverse events have been observed in about 1% of treated patients.1 Known ocular adverse events associated with nivolumab include cranial nerve six and seven palsy, myasthenia gravis, conjunctivitis, corneal graft rejection, dry eye, keratitis, uveitis, macular edema, immune retinopathy affecting photoreceptors, and Vogt-Koyanagi-Harada (VKH)-like syndrome.1,2
Including this case, there are ten single case reports of nivolumab induced VKH-like syndrome (nVKH).3-11 Affected patients had a median age of 62.0 years (Range = 35.0 to 74.0 years; Mean = 59.7 years). Three subjects were male and seven were female (M:F ratio 0.43:1). A total of five (50%) patients were being treated with nivolumab for metastatic cutaneous melanoma, three (30%) for metastatic renal cell carcinoma, one patient each (10%) for metastatic non-small cell lung cancer, hypopharyngeal cancer, and choroidal melanoma. Nivolumab was the only checkpoint inhibitor being used in eight (80%) patients, with two (20%) patients also receiving ipilimumab concurrently. Patients presented with nVKH findings at a median of 2.0 months (Range = 1.0 to 24.0 months; Mean = 5.1 months). A total of eight (80%) patients had bilateral involvement, and two (20%) had unilateral involvement. Anterior segment findings included anterior uveitis (6/8, 75%), keratic precipitates (3/8, 38%), posterior synechiae (3/8, 38%), and iris nodules (1/8, 13%). Initial posterior segment findings included serous retinal detachment (9/10, 90%), chorioretinal folds (8/10, 80%), vitreous opacities (4/10, 40%), optic disc edema (3/10, 30%), cystoid macular edema (1/10, 10%), and bacillary layer detachment (1/10, 10%). The non-ocular VKH findings of poliosis (3/10, 30%), headache (2/10, 20%), vitiligo (1/10, 10%), and hearing loss (1/10, 10%) were reported in a minority of patients. One patient with both poliosis and vitiligo had undergone treatment with combination therapy of ipilimumab and nivolumab. In total, nine patients (90%) with nVKH experienced resolution of their ocular inflammation with systemic corticosteroids, while one (10%) only needed topical corticosteroids. One patient (10%) underwent vitrectomy for persistent vitreous opacities. Choroidal depigmentation with a sunset glow fundus was described in five (83%) patients with available longitudinal follow up information, while one (17%) patient developed hyperpigmentation in the macula from RPE changes.
A bacillary detachment is charactered by accumulation of intra-retinal fluid resulting in splitting of the retina at either the myoid zone, ellipsoid zone, or IS/OS junction.12 This phenotype of retinal exudation was first described in a case of macular toxoplasmosis with pachychoroid, but has since been reported in other variants of posterior uveitis and pachychoroid disease, as well as in patients with choroidal neovascularization, ocular trauma, and choroidal metastasis.12 Formation of a bacillary detachment may result from hyper-acute choroidal exudation followed by forceful intraretinal exudation that results in splitting within the photoreceptor layer.12
The prior understanding of nVKH suggested it was generally a simultaneous and bilateral condition observed within five months of drug initiation, manifesting as bilateral anterior uveitis, diffuse choroidal thickening with chorioretinal folds and often only minimal or modest overlying subretinal fluid.3-10 In contrast, our patient developed visual symptoms two years into her course of nivolumab with a unilateral, extra-macular, and creamy chorioretinal lesion characterized by bacillary layer detachment with localized choroidal thickening on OCT, and no evidence of anterior segment inflammation. The combination of these atypical findings and the presence of an irregular (lumpy bumpy) anterior choroidal contour with accumulation of subretinal fluid and overlying disruption of the RPE, interdigitation zone, and ellipsoid layer, all features commonly observed on OCT in those with choroidal metastases,13 prompted the initial investigation for possible recurrence of malignancy with metastatic spread to the choroid. Our suspicion for drug-induced ocular inflammation began when the patient developed a second creamy chorioretinal lesion in the right eye along with new choroidal thickening with chorioretinal folds in the left eye, and was further bolstered when retrospective chart review revealed that incidental discontinuation of one dose of nivolumab by the patient’s oncologist preceded the spontaneous resolution of the first chorioretinal lesion in her right eye.
While not fully understood, the pathogenesis of nVKH can be inferred by nivolumab’s mechanism of action and our current knowledge of VKH. The immune activation induced by nivolumab likely incites lymphocytic infiltration of the uvea, leading to choroidal thickening and inflammation.3 Over time, the ensuing choroidal edema and choriocapillaris insufficiency damages the RPE enough to result in the accumulation of subretinal and intraretinal fluid.3 The asynchronous development of nVKH in our patient’s eyes appears to support this theory, as the less affected left eye possessed a similarly thickened choroid with chorioretinal folds, but no evidence of intra- or subretinal fluid. The silver lining for patients with nVKH is that this adverse event reflects a robust immune response, possibly to their underlying malignancy, and may indicate a more favorable long-term prognosis since paraneoplastic VKH-like uveitis has been associated with longer survival times in metastatic malignancy.14
Adapted from: Ng CC, Ng JC, Johnson RN, McDonald HR, Agarwal A. Nivolumab-Induced Harada-like Uveitis with Bacillary Detachment Mimicking Choroidal Metastasis. Accepted for publication at Retinal Cases & Brief Reports 2021