May, 2022
Presented by Braden Burckhard, MD


Presented by Braden Burckhard, MD

A 61-year-old Caucasian, asymptomatic male was referred due to abnormalities in his peripheral retina.
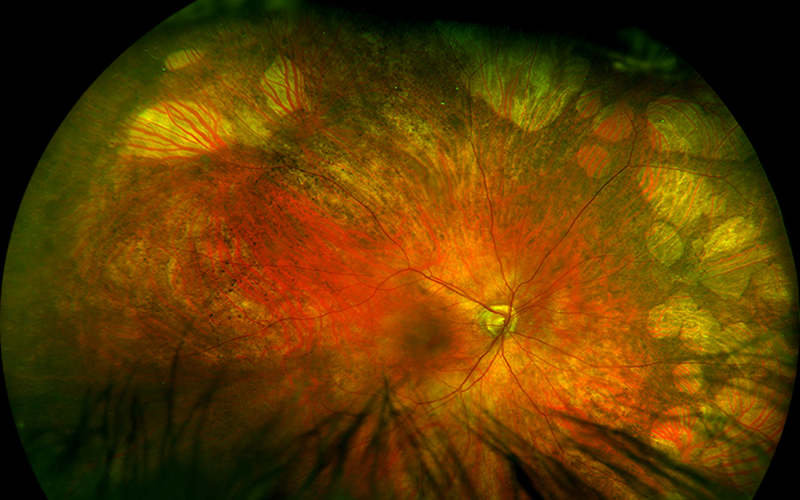
Figure 1A: Wide-field color photo of the right eye. Note the extensive areas of reticular RPE clumping and numular areas of atrophy in the peripheral retina.
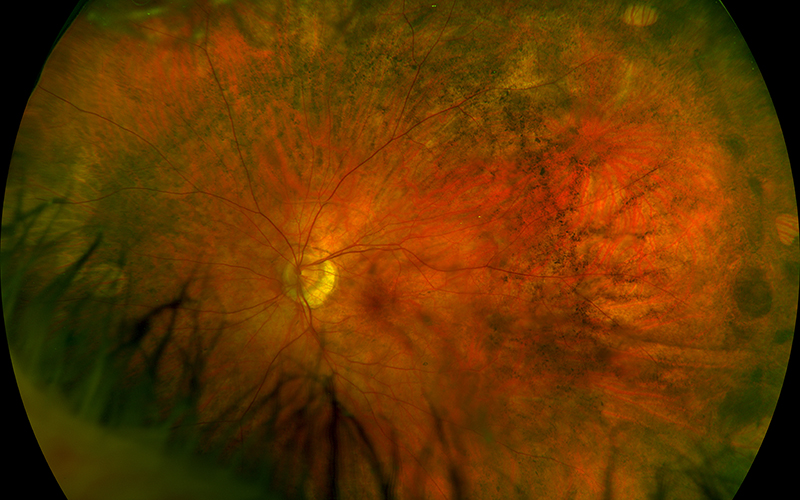
Figure 1A: Wide-field color photo of the left eye. Note the peripheral areas of reticular RPE clumping and numular atrophy.
The patient was found by his optometrist to have retinal pigmented epithelial (RPE) changes in the peripheral retina in both eyes that were not previously documented. The patient had not noted any changes in his central, peripheral, color, or night vision. He had no significant past ocular history except for high myopia of about 10 diopters in both eyes. His past medical history was significant for a 30 year history of HIV for which he takes dolutegravir/lamivudine. None of his family members have ever been diagnosed with hereditary or peripheral retinal disorders. His review of systems was negative.
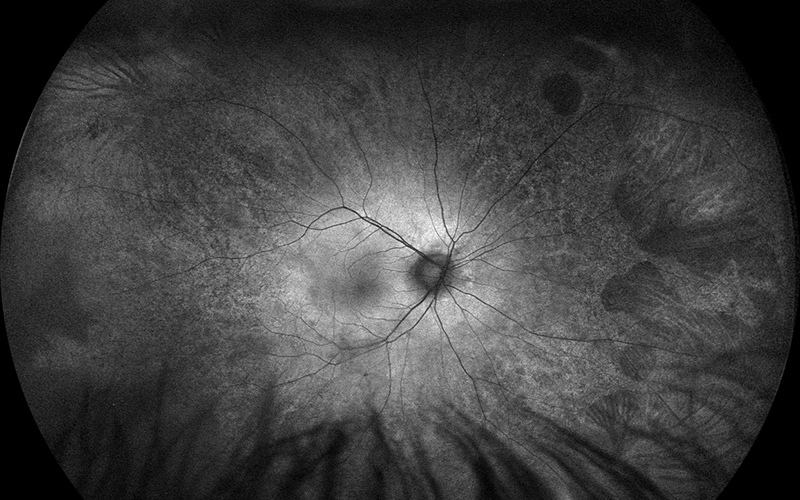
Figure 2A: Wide-field fundus autofluorescence of the right eye. Note the peripheral areas of reduced autofluorescence, particularly in the nasal retina and far temporal periphery.
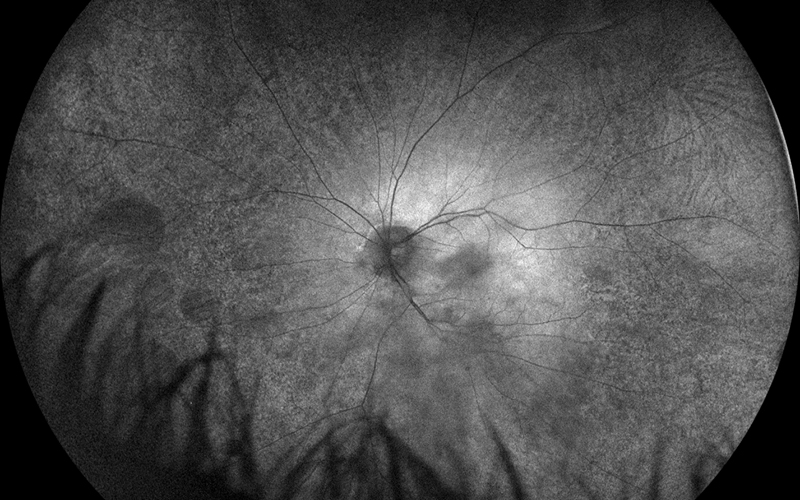
Figure 2A: Wide-field fundus autofluorescence of the left eye. Note the peripheral areas of reduced autofluorescence, particularly in the nasal retina.
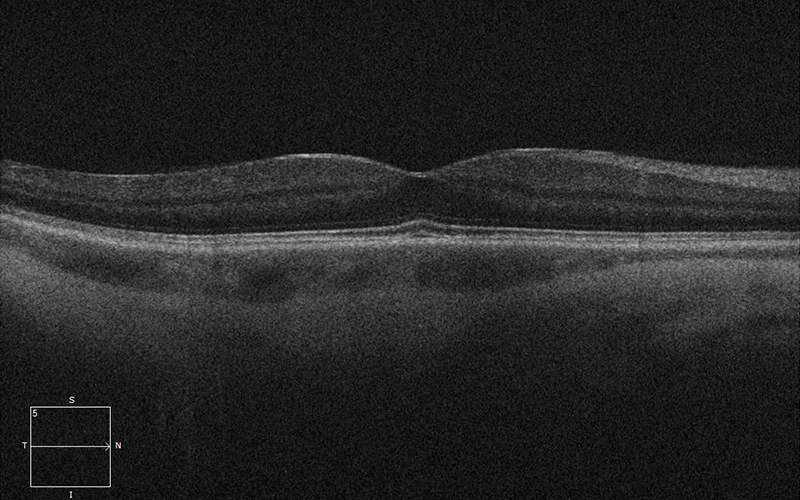
Figure 2A:SD-OCT of the right eye is unremarkable.
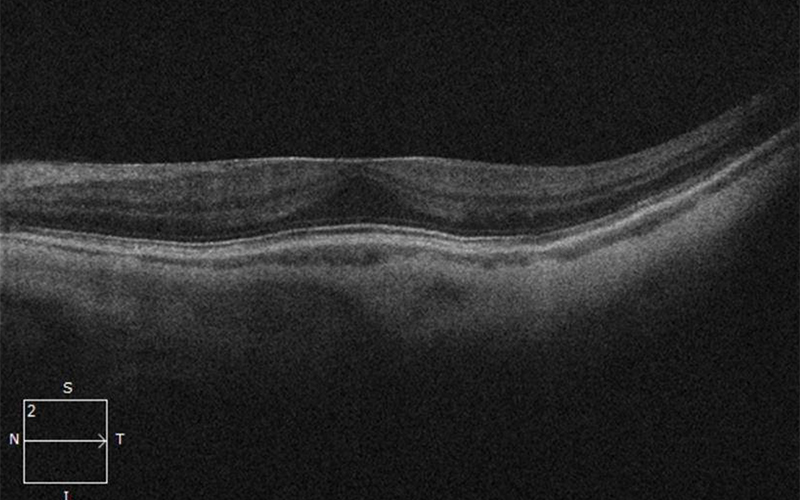
Figure 2A:SD-OCT of the left eye is unremarkable except for a mild epiretinal membrane.
His best corrected Snellen visual acuity measured 20/25 in the right eye (OD) and 20/25 in the left eye (OS). The intraocular pressure was 20 mmHg in the right eye and 14 mmHg in the left eye. Anterior segment examination was unremarkable. Examination of the right eye showed reticular pigment changes as well as large nummular areas of RPE atrophy in both eyes that was more prominent in the right eye as compared with the left eye (Figures 1A and 1B). Fundus autofluorescence (FAF) showed hypoautofluorescence of the lesions in both eyes (Figures 2A and B). Spectral domain optical coherence tomography (SD-OCT) of both eyes showed no significant abnormalities (Figure 3A and B).
Differential Diagnosis
After further discussion with the patient, he reported prior treatment with didanosine for HIV. He began the medication 20 years ago and took 400 mg daily for about one year before switching to alternative therapy
Discussion
Didanosine was one of the earliest nucleoside reverse transcriptase inhibitors (NRTI) used in combination treatment of HIV infection. Over the past 35 years, it has been an integral part of the highly active antiretroviral therapy (HAART). A phase I/II study of HIV infected children who were treated with didanosine revealed that 4 individuals developed didanosine related retinal toxicity.1 Infrequently, there have been other reported cases.2,3
The largest case series of didanosine retinal toxicity described bilateral, symmetric, concentric mid-peripheral chorioretinal atrophy generally sparing the posterior pole.3 These atrophic changes often start as retinal pigment epithelium (RPE) mottling and progress to nummular patches of atrophy. Fundus autofluorescence (FAF) imaging typically reveals mid-peripheral hypo-autofluorescence consistent with the areas of atrophy. Fluorescein angiography (FA) similarly shows hypo-fluorescence in the areas of atrophy. Due to mid-peripheral nature of the toxicity, patients may be asymptomatic and diagnosis may be delayed. Electroretinogram testing may reveal severe reduction to nearly extinguished responses in photoreceptor function. Despite cessation of didanosine, progression of retinal damage can occur similar to what is seen with Mellaril toxicity. This has been attributed to the possible additive effect of other NRTI class drugs.
Due to issues of multiple toxicities, drug interactions, virologic efficacy, and cost, it has been suggested that didanosine should be removed from the HAART regimen.4 Didanosine is no longer recommended by the Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV for use in children due to higher rates of adverse effects than other NRTIs.5