Sept, 2020
Presented by Caleb Ng, MD


Presented by Caleb Ng, MD

A 34-year-old Caucasian man with 2 weeks of central photopsias, para-central scotomata, and a grey haze affecting his right eye.
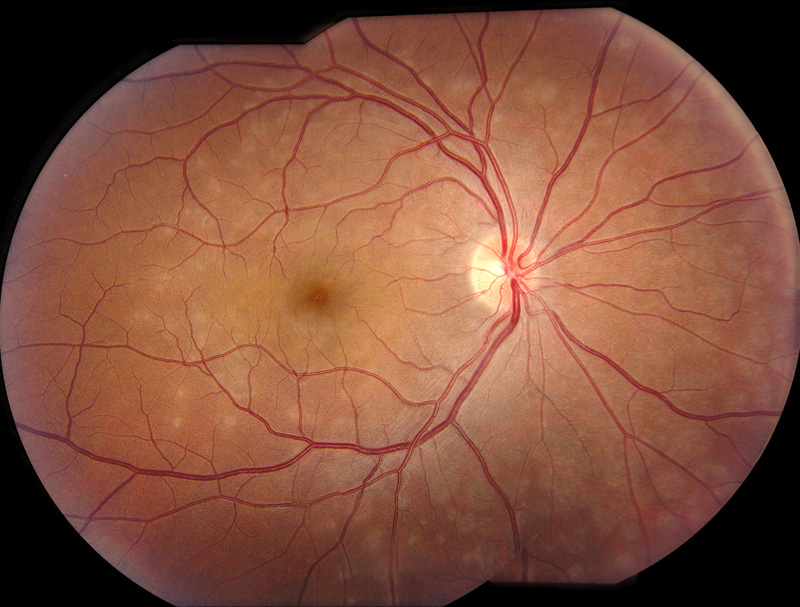
Figure 1: Fundus photo of the right eye. Note the extenive areas of deep pale grayish circular spots scattered through out the macula and nasal to the nerve.
His past medical and surgical histories were unremarkable. He previously had undergone myopic-photorefractive keratectomy. On examination his Snellen visual acuity was 20/20+2 on the right and 20/ 13-1 on the left. Intraocular pressure was 18 mmHg on the right and 14 mmHg on the left. Pupils, extraocular motility, and confrontation visual fields were unremarkable. Anterior segment examination was normal. Examination of the right posterior segment was notable for mild foveal granularity and small, grey, outer-retinal lesions centered around the optic nerve with extension to the mid-periphery (Fig. 1). The posterior segment of the left eye was normal. Spectral domain (SD-OCT) tomography in the area of the grey retinal lesions revealed focal areas photoreceptor disruption (Fig. 2). Fundus autofluorescence revealed widespread areas of hyper-autofluorescence of variable size (Fig. 3); Fluorescein angiography showed multiple circular areas of punctate hyperfluorescence of approximately 300-500um in diameter surrounding the optic nerve and, in the macula, (Figure 4A) that persisted in the late phases without leakage (Figure 4B). Late phase Indocyanine green angiography late hypofluorescence (Fig. 5).
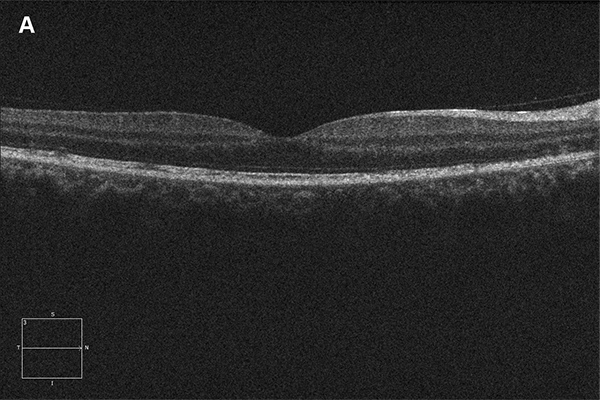
Figure 2A and B: Horizontal SD-OCT scan through macula. Note the areas of disruption of the photoreceptors. Figure 2B is an en face view of the macula at the level of the photoreceptors, showing this disruption.
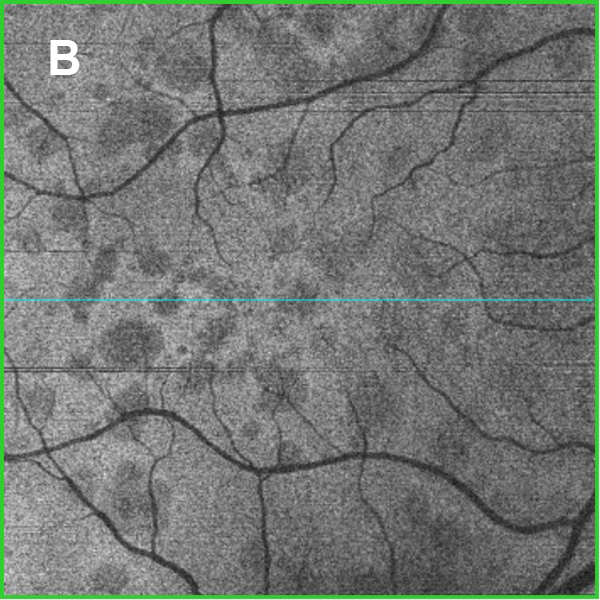
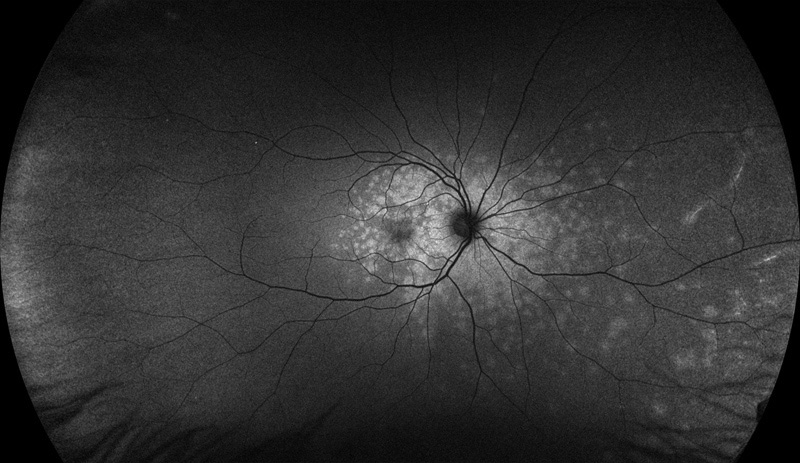
Figure 3: Wide-field fundus autofluorescence of the right eye. Note the extensive areas of increased autofluorescence mostly centered around the macula and nerve.
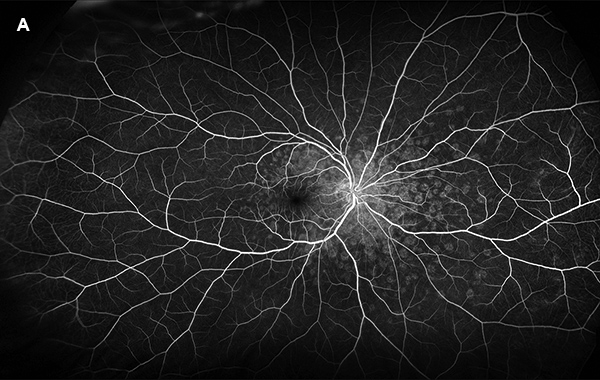
Figure 4A: Wide-field fluorescein angiogram, early phase, right eye. Note the extensive areas of increased fluorescence in the macula and nasal retina corresponding to the pale lesions seen on the color photo (Figure 1).
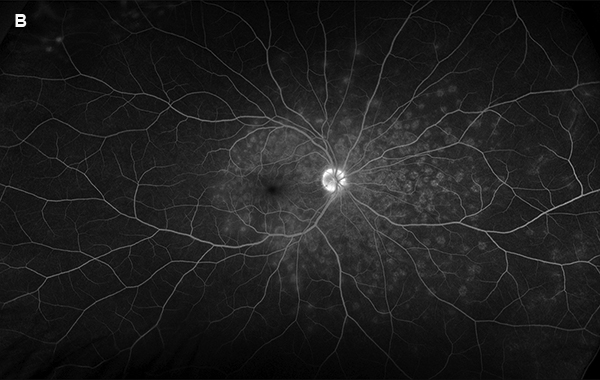
Figure 4B: Wide-field fluorescein angiogram, late phase, right eye. The extensive areas of increased fluorescence are seen without leakage in the late phase.
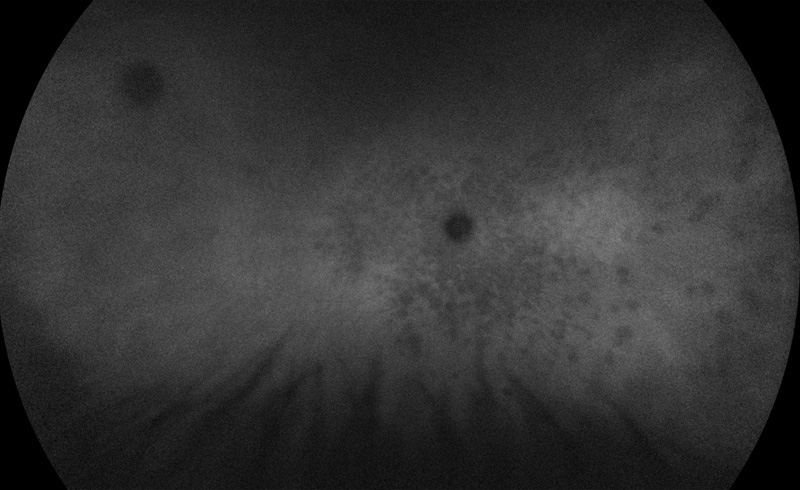
Figure 5: Wide-field indocyanine angiogram of the right eye. Numerous areas of circular hypofluorescence are seen that correspond to the pale areas on the color photo and the areas of increased autofluorescence and increased fluorescence on the fluorescein angiogram.
Differential Diagnosis
Additional History and Diagnosis
Upon further inquiry, our patient denied any preceding viral symptoms, but did reveal that he received Flucelvax Quadrivalent® influenza vaccine (Seqirus, Holly Springs, North Carolina) two weeks prior to onset of symptoms. Collectively, the history, and clinical and multimodal imaging findings lead to the diagnosis of MEWDS following influenza vaccination.
Discussion
In 1984, Jampol and colleagues described a new chorioretinal disorder name Multiple Evanescent White Dot Syndrome (MEWDS).1 While there have been no known racial or hereditary predilections, the disease is most commonly diagnosed in young to middle-aged women. As many as 30% of affected patients report a viral prodrome. MEWDS is almost always a unilateral disease, and common symptoms include photopsias, dyschromatopsia, or paracentral scotomata. The disorder is thought to have a benign prognosis with spontaneous resolution after two to three months.2 To the best of our knowledge, there have been nine published cases of MEWDS following various vaccinations, including rabies,3 human papilloma virus,4,5 hepatitis A,6,7 hepatitis B,8 meningococcal,4 Yellow fever,6 and influenza.9-11 Collectively, patients with post-vaccine MEWDS tended to be healthy (77.8%), young to middle age (median 33 years; mean 31.7 years; range 16–53 years) women (66.7%). Racial classification was 44.4% Caucasian, 22.2% Asian, and 33.3% undisclosed. Symptoms manifested on average 13.3 days (median: 14; range: 1-30) after immunization. Mean presenting Snellen visual acuity was of 20/38 (median: 20/25-2, range: 20/16 to 20/200). Patients most commonly described photophobia (88.9%), followed by central or paracentral scotomata (44.4%) and dyschromatopsia (33.3%). Seven cases (77.8%) of post-vaccine MEWDS displayed spontaneous resolution back to baseline Snellen visual acuity over an average of 6.9 weeks (median: 6 weeks; range: 4–12 weeks). The patient with MEWDS following rabies vaccination refused oral corticosteroids, but agreed to receive peri-ocular corticosteroid as part of the treatment protocol. Ogino and colleagues described a case of MEWDS following human papilloma virus vaccine that appeared to have resolved without treatment in two months. Subsequently, the patient noted progressive peripheral vision loss over two years. Repeat imaging revealed mid-peripheral vascular leakage on fluorescein angiography. Due to an allergy to methylprednisolone, she was treated with high dose betamethasone and anti-histamines, leading to drastic reduction in leakage on FA. Unfortunately, the leakage recurred as patient was weaned off the corticosteroid. Given the atypical symptoms and examination findings, the authors discussed the possibility of a different concurrent disease entity, such as acute zonal occult outer retinopathy. Visual acuity at last visit in affected eyes had mean of 20/18.5, with median of 20/20 and range of 20/16 to 20/20.
The exact pathogenesis of MEWDS is yet to be fully elucidated. Most recent hypotheses suggest an immune-mediated mechanism occurring at either the outer retina,1,2,12 the choriocapillaris/inner choroid,13-15 or both in genetically predisposed individuals, and the occurrence of MEWDS following vaccination would tend to support such theories. Specifically, vaccines have been suggested to trigger an inflammatory response resulting in uveitis by means of molecular mimicry, or direct antigen-mediated cellular/humoral immune response, adjuvant-mediated inflammation.16,17 As many as 160 million doses of influenza vaccine were administered in the United States for the 2019–2020 season,18 and billions more have been given since the first report of vaccine-associated MEWDS in 1996. It remains possible, therefore, that the occurrence of MEWDS following immunization is coincidental. However, given the generally mild and self-resolving nature of MEWDS, it could also be that post-vaccination cases of MEWDS tend to go unrecognized and unreported. The benefits of continuing to follow established vaccination guidelines far outweigh the risks of uveitis for the vast majority of patients.17
Adapted from: Ng CC, Jumper JM, Cunningham Jr ET. Multiple evanescent white dot syndrome following influenza immunization-A multimodal imaging study. American Journal of Ophthalmology Case Reports. 2020 Aug 3:100845.