Sept, 2021
Presented by Braden Burckhard, MD


Presented by Braden Burckhard, MD

A 60 year-old female presented with a 3 month history of visual disturbances in both eyes.
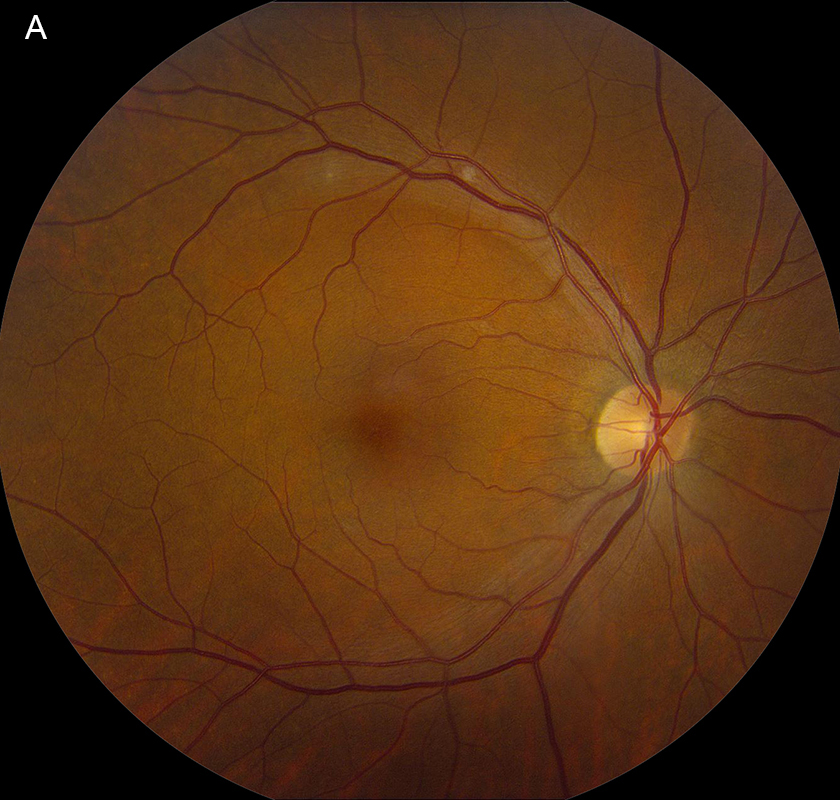
Figure 1A: Color photo of the right retina. Note the 2 areas of faint retinal whitening along the superotemporal arcade representing fading cotton-wool spots.
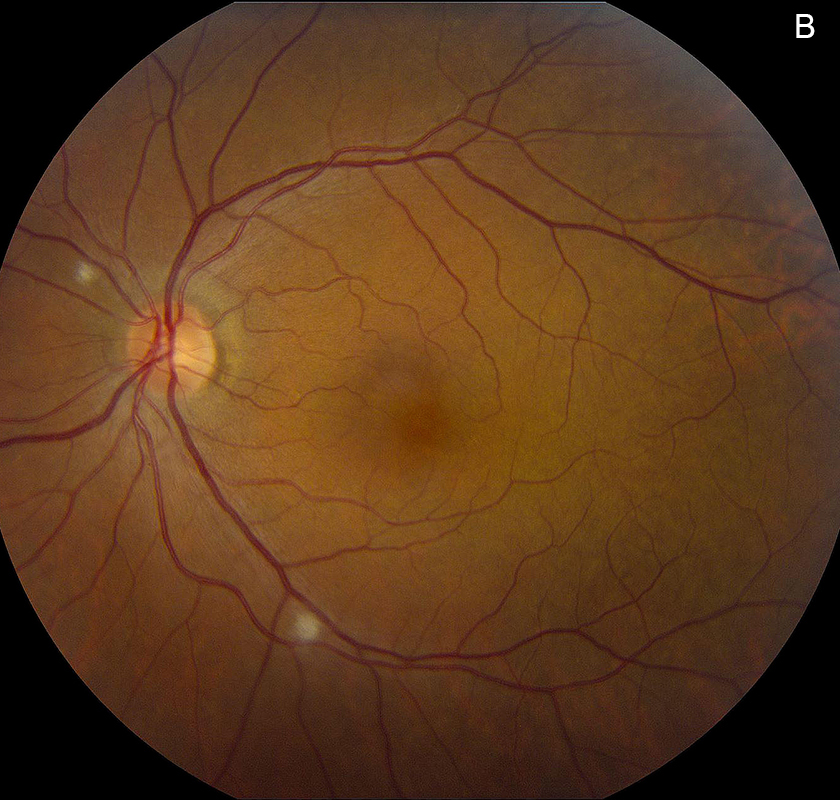
Figure 1B: Color photo of the left retina. Note the cotton-wool spot superonasal to the nerve and along the inferotemporal arcade.
The patient noticed increased floaters for the past couple weeks in addition to occasional bright spots in her vision which she described as “looking through a kaleidoscope.” She had a history of ocular migraines for the last 3 months which she thinks may be associated. Her review of systems was unremarkable except for a history of deep venous thrombosis (DVT) in her left leg which occurred one month prior to her presentation. She was currently on Xarelto. She was previously vaccinated for COVID-19, having received both doses 3-4 months earlier. Her past medical history was otherwise noncontributory. She denied the use of any illicit substances but does admit to being a former smoker (quit in 2012). Her past ocular history is unremarkable.
Her Snellen visual acuity measured 20/16 in the right eye (OD) and 20/20 in the left eye (OS). Her intraocular pressure was 12 mmHg OD and 14 mmHg OS. Anterior segment examination was unremarkable OU. The funduscopic examination was noteworthy for 2 cotton-wool spots in each eye. (Figures 1A and B). Spectral domain optical coherence tomography (SD-OCT) showed normal retinal architecture in both eyes (Figures 2A and B. Fluorescein angiography was performed, demonstrating trace hyperfluorescence along the inferotemporal arcade in the left eye, corresponding to the cotton-wool spot. The right eye angiogram was unremarkable (Figures 3A and B).
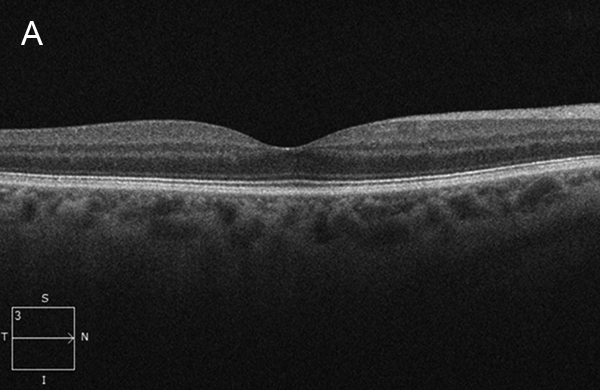
Figure 2A: Spectral-domain OCT of the right macula shows a normal study.
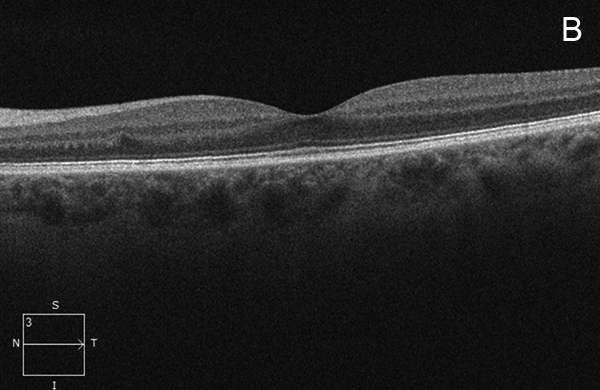
Figure 2B: Spectral-domain OCT of the left macula shows a normal study.
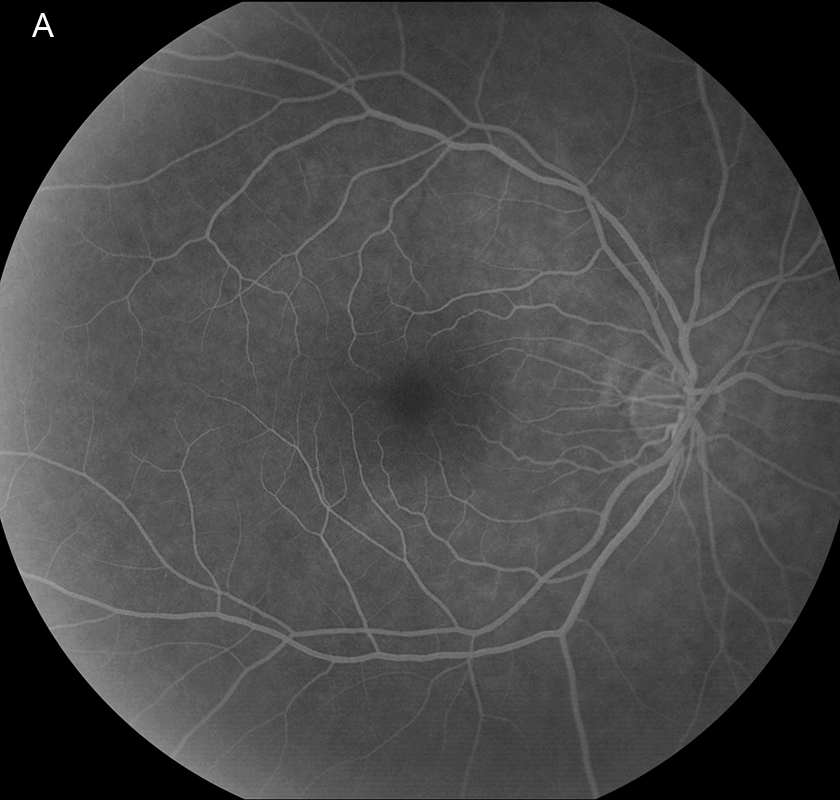
Figure 3A: Late-phase fluorescein angiogram of the right eye. Note the absence of abnormal fluorescence.
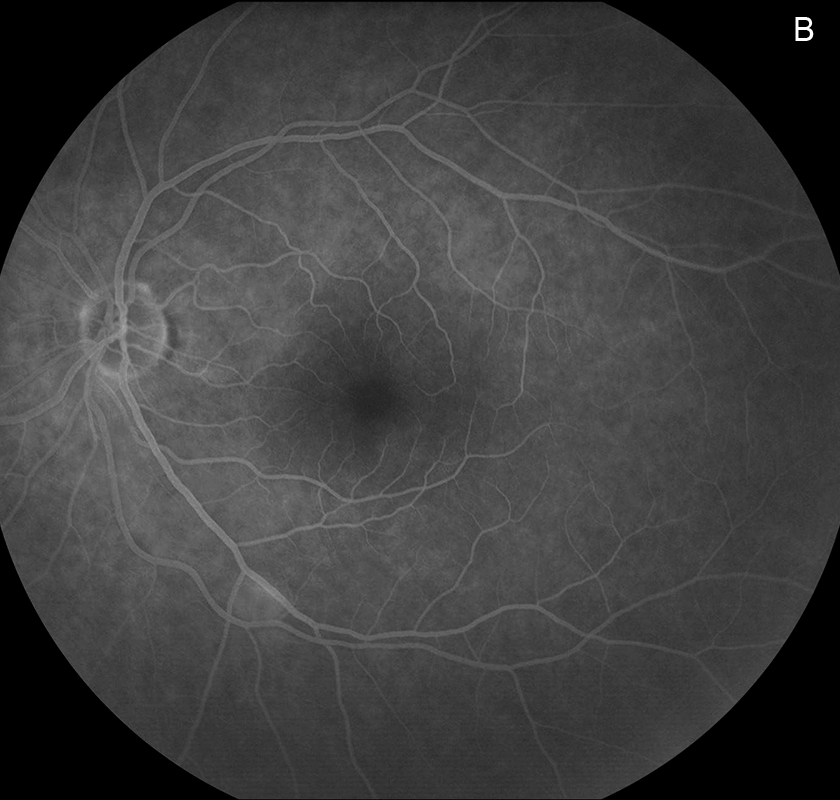
Figure 3B: Late-phase fluorescein angiogram of the left eye. Faint staining of the cott-wool spot along the inferotemporal arcade is present.
Differential Diagnosis
A broad differential diagnosis for cotton wool spots may be seen at the following link: West Coast Retina Case of the Month Aug, 2019
Additional History, Evaluation and Diagnosis
After the patient was evaluated in the emergency department for her DVT, she was started on Xarelto and followed up with hematology 12 days later. The etiology of the thrombosis was attributed to immobility from prolonged sitting while on recent teleconference phone calls. The patient was to remain on Xarelto for three months before discontinuing the medication after repeat ultrasound of the lower extremities. The patient followed up with her primary care provider two weeks later and noted occasional bright lights in her vision as well as intermittent left-sided chest pain over the past seven days. An EKG was obtained which showed no changes from previous studies. She presented to our retina clinic approximately one week later to address her ocular symptoms. No laboratory testing had been performed to work up the DVT prior to her eye exam. Due to the presence of cotton wool spots on examination and recent DVT, the following labs were obtained: CBC with differential, CMP, ESR, ANA, Factor V Mutation, Protein S, Protein C, Anticardiolipin, Serum homocysteine, Antiphospholipid antibodies, prothrombin time, partial thromboplastin time, and a cholesterol panel. Most of these labs returned within normal limits except for the patient’s hemoglobin level which was slightly decreased at 11.2 g/dL (11.7-15.5 g/dL) and the prothrombin time which was slightly elevated 15.5 sec (12.1-14.7 sec).
One week after her retinal evaluation, the patient was seen by Cardiology. At this visit, she reported having mild fatigue and increased shortness of breath with activity. A chest CT-angiogram (CTA) was ordered at this point to rule out pulmonary embolism (PE). The CTA was obtained later that day without evidence of a pulmonary embolism, however, there was an elongated and spiculated mass-like density noted in the apex of the right lung measuring approximately 3.5 x 2.3 x 1.9 cm, concerning for possibly malignancy (Figure 4). These findings warranted further workup with a biopsy of the mass, which was performed three weeks later. The biopsy results returned positive for adenocarcinoma of the lung. A PET scan was performed shortly after the biopsy, showing metastasis to local lymph nodes, but no evidence of spread to the abdomen, pelvis, or bones. The patient will be seen by Oncology for further management.

Figure 4: CT-Angiogram of the chest. Note the spiculated mass in the right lung.
She was recently seen for follow up regarding her retinal findings with complete resolution of the cotton wool spots and no other changes in her ophthalmic exam.
Discussion
Cotton wool spots (CWSs) result from an ischemic event or occlusion of pre-capillary arterioles, leading to infarction of the retinal nerve fiber layer.1 More specifically, they occur from focal interruption of axoplasmic flow in the retinal nerve fiber layer and the accumulation of intra-axonal organelles.2 They most commonly appear as slightly elevated, white, feathery-bordered lesions of the inner retina. CWSs may become symptomatic if they are near the fovea and typically resolve within 6-12 weeks.3 The differential for cotton wools spots is broad and the patient’s entire medical history must be considered to narrow the possible etiologies. Brown et al analyzed 24 consecutive patients with incidental findings of cotton wool spot and uncovered the following: undiagnosed diabetes mellitus (21%), systemic hypertension (21%), cardiac valvular disease (8%), radiation retinopathy (8%), severe carotid artery obstruction (8%), and dermatomyositis, systemic lupus erythematosus, polyarteritis nodosa, leukemia, AIDS, Purtscher’s retinopathy, metastatic carcinoma, intravenous drug use, partial central retinal artery obstruction, and giant cell arteritis (4% each).1 Our patient was a normotensive, non-diabetic, mid- to upper-aged female. Due to the lack of obvious risk factors for CWSs, an in-depth workup was warranted. With recent hisory of DVT, many labs were ordered, including hematologic and coagulation studies, which showed a slight decreased hemoglobin and slightly elevated prothrombin time. Her cumulative clinical picture led to further systemic workup and eventually revealed adenocarcinoma of the lung.
Hypercoagulable or prothrombotic state of malignancy occurs due to the ability of tumor cells to activate the coagulation system. Prothrombotic factors in cancer include the ability of tumor cells to produce and secrete procoagulant/fibrinolytic substances and inflammatory cytokines, and the physical interaction between tumor cell and blood (monocytes, platelets, neutrophils) or vascular cells4. Other mechanisms of thrombus promotion in malignancy include nonspecific factors such as the generation of acute phase reactants and necrosis (i.e., inflammation), abnormal protein metabolism (i.e., paraproteinemia), and hemodynamic compromise (i.e., stasis).3
Thrombosis is a serious and prevalent complication of malignancy as it represents the second most frequent cause of death in cancer patients.5 Additionally, cancer patients represent 20% of all patients in whom DVT and PE are diagnosed. A hypercoagulable state may lead to other systemic complications, as well as DVT, as seen in our patient. Clinically detectable venous thromboembolism (VTE) is present in 15% of all cancer patients.7,8 Even in the absence of obvious thrombosis, cancer patients with solid tumors and leukemias commonly present with abnormal laboratory coagulation tests, characterized by varying degrees of clotting activation, indicating a subclinical hypercoagulable condition.9
Several factors involved in the immune response to neoplasia, such as the development of acute phase reactants, abnormal protein metabolism, necrosis, and hemodynamic rearrangements, can all contribute to the overall activation of blood coagulation in cancer patients.3 Interestingly, tumors often produce vascular endothelial growth factor (VEGF) which increase vascular permeability and increase platelet activation and adhesion4. This mechanism, in addition to the others outlined by Caine,4 lead to an overall increased pro-thrombotic state.
The development of CWSs in cancer patients may be a result from several hematologic abnormalities including venous thromboembolism, individual thrombotic events/vein occlusions, or hemostasis from increased blood viscosity. The cotton wool spots found in our patient may easily be explained by her history of mild anemia, increased prothrombin time, and adenocarcinoma of the lung. Her management will involve close observation with serial retinal exams, as well as treatment of underlying disease by Hematology and Oncology.