June, 2023
Presented by Malini Pasricha, MD


Presented by Malini Pasricha, MD

A 32-year-old man presents with blurry vision of the left eye for unknown duration.
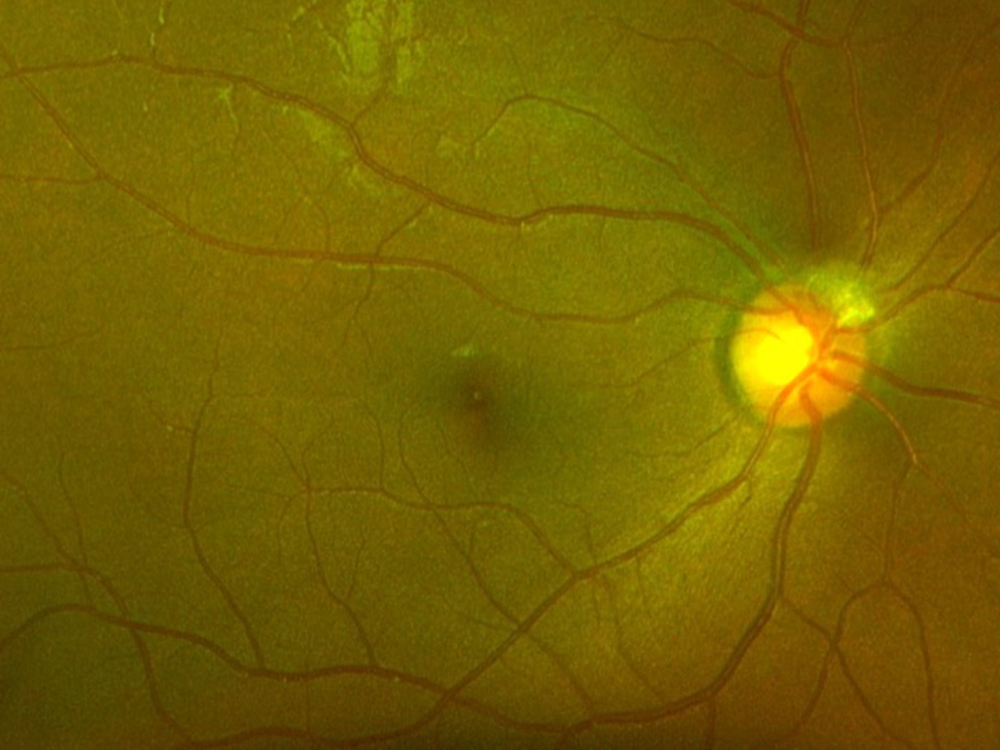
Figure 1A: Color photo of the right eye. There is some mild arteriolar narrowing but the disc and macula are normal.
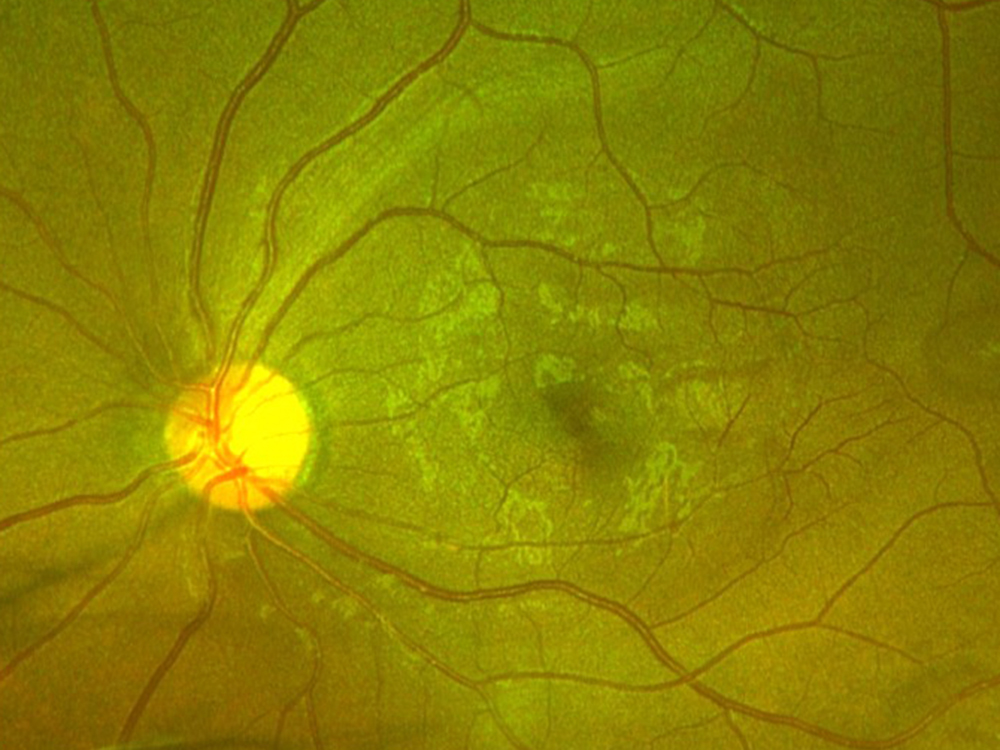
Figure 1B: Color photo of the left eye. Note the temporal retina with subtle areas of darker appearing retina.
The patient reports some floaters in the left eye. He denied any flashes, scotoma, pain, or headaches. His past ocular history was significant for lattice degeneration of the retina in both eyes. The patient’s best corrected Snellen visual acuity measured 20/20 OD and 20/160 OS. Intraocular pressures were normal. Anterior segment exam was normal. The macular exam of both eyes is shown in Figures 1A and B. The OCT angiogram showed some dropout of the superficial capillary plexus in each eye, but more prominently in the left (Figures 2A and B). Horizontal OCT through the macula of both eyes demonstrated temporal inner retinal thinning in the left eye (Figures 3A and B).
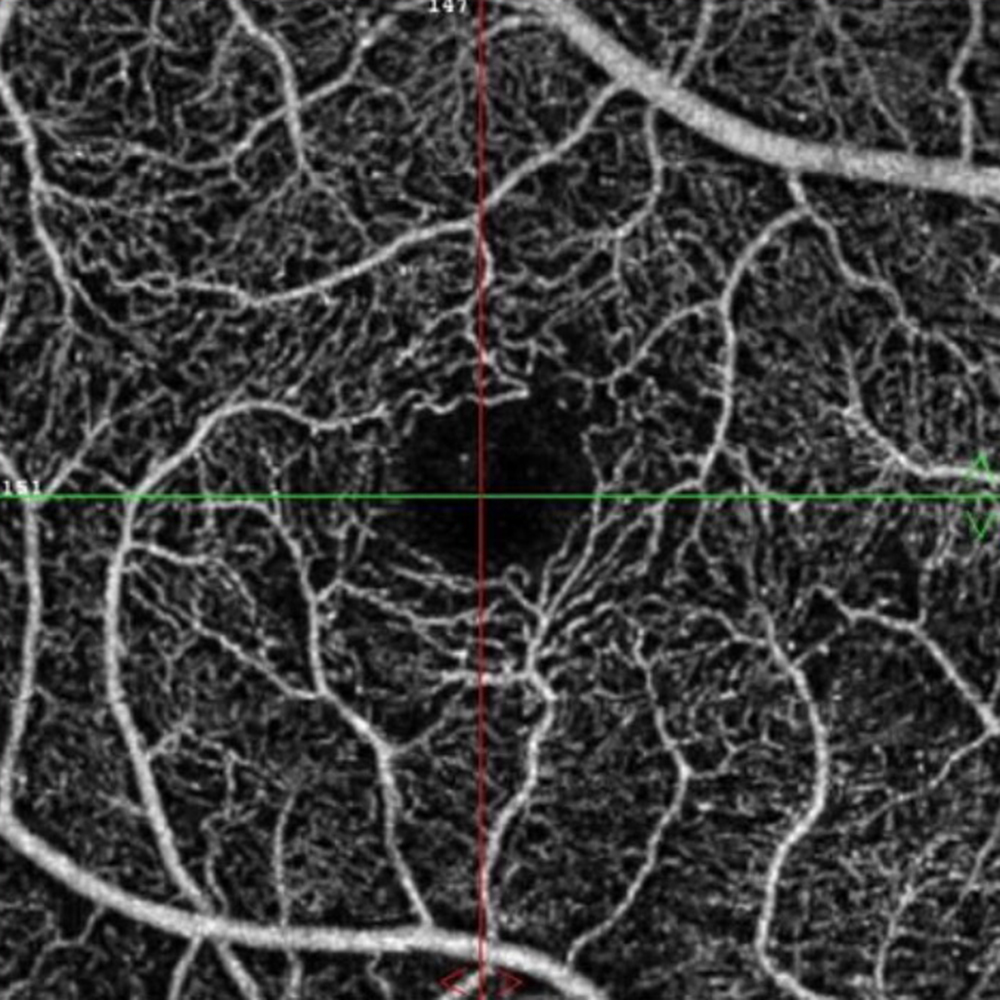
Figure 2A: OCT Angiogram of the right macula. Subtle capillary loss is present.
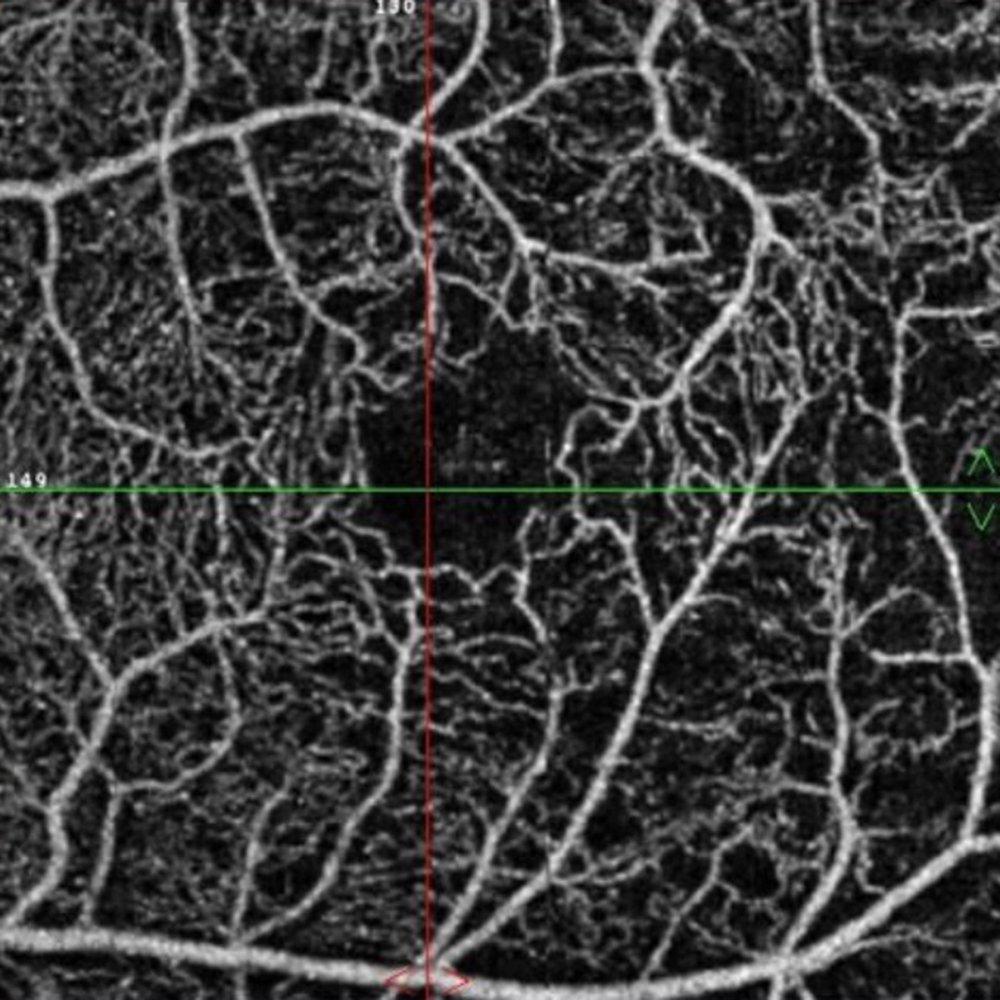
Figure 2B: OCT angiogram of the left macula. Note the capillary drop-out
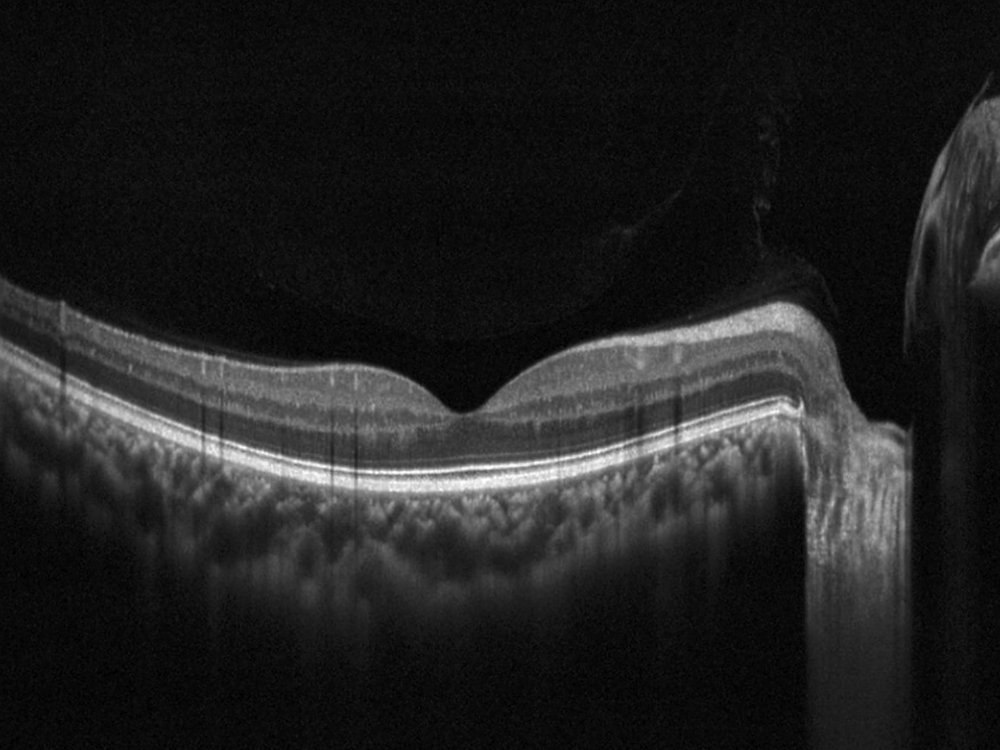
Figure 3A: Horizontal OCT scan of the right macula. The study is mostly normal with perhaps a subtle thinning temporally.
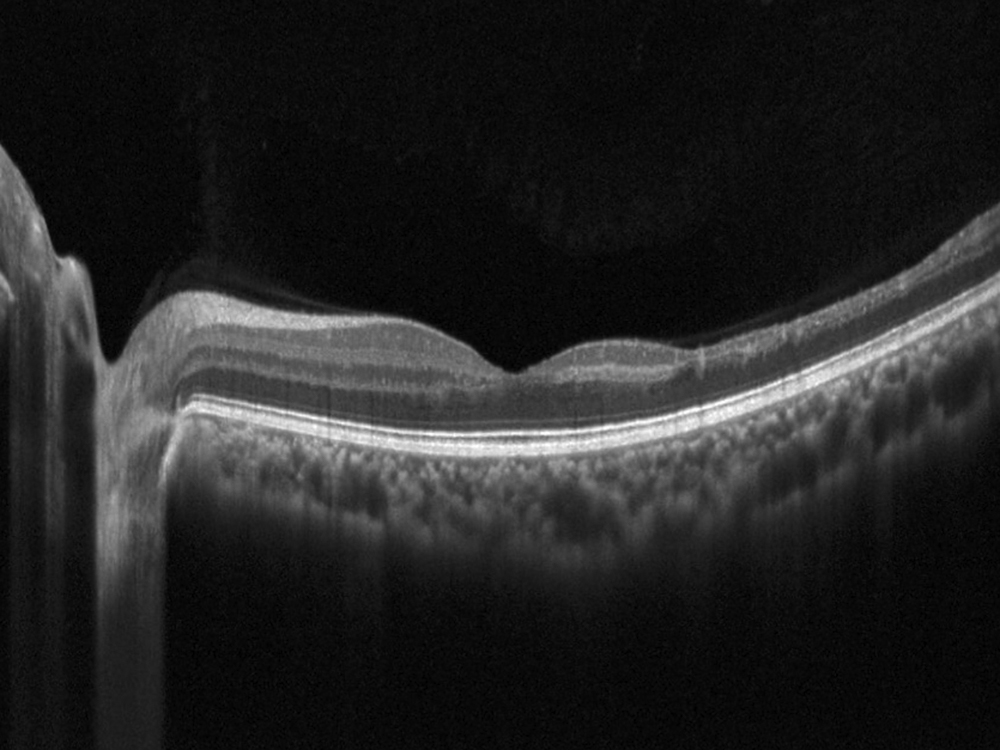
Figure 3B: Horizontal OCT scan of the left macula. Note the marked thinning of the temporal macula.
Differential Diagnosis
Macular Ischemia
The patient’s past medical history was significant for sickle cell anemia (Hgb SS). Wide field color photos demonstrated round yellow lesions inferior to the optic nerve in the eye, consistent with old salmon patch hemorrhages (Figures 4A and B). Wide field fluorescein angiogram photos demonstrated blockage in the area of the old salmon patch hemorrhages in the left eye, and peripheral non-perfusion in both eyes; there was no obvious neovascularization (Figures 5A and B). Wide field fundus autofluorescence photos demonstrated hyper-autofluorescence (due to hemoglobin breakdown products) at the site of the salmon patch hemorrhages (Figures 6A and B).
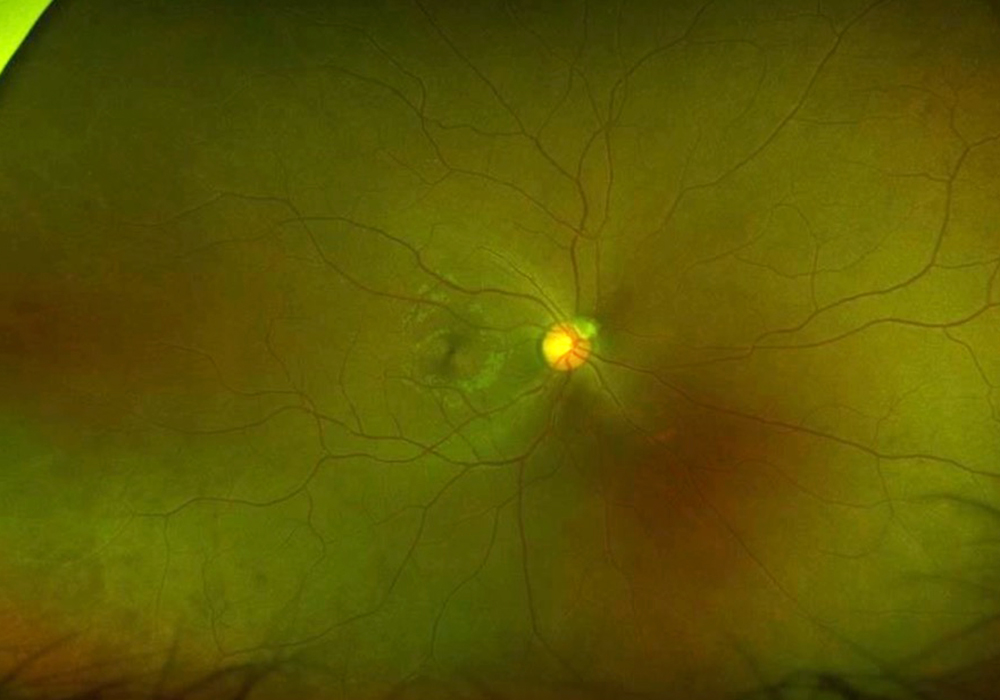
Figure 4A: Wide-field color photograph of the right eye.
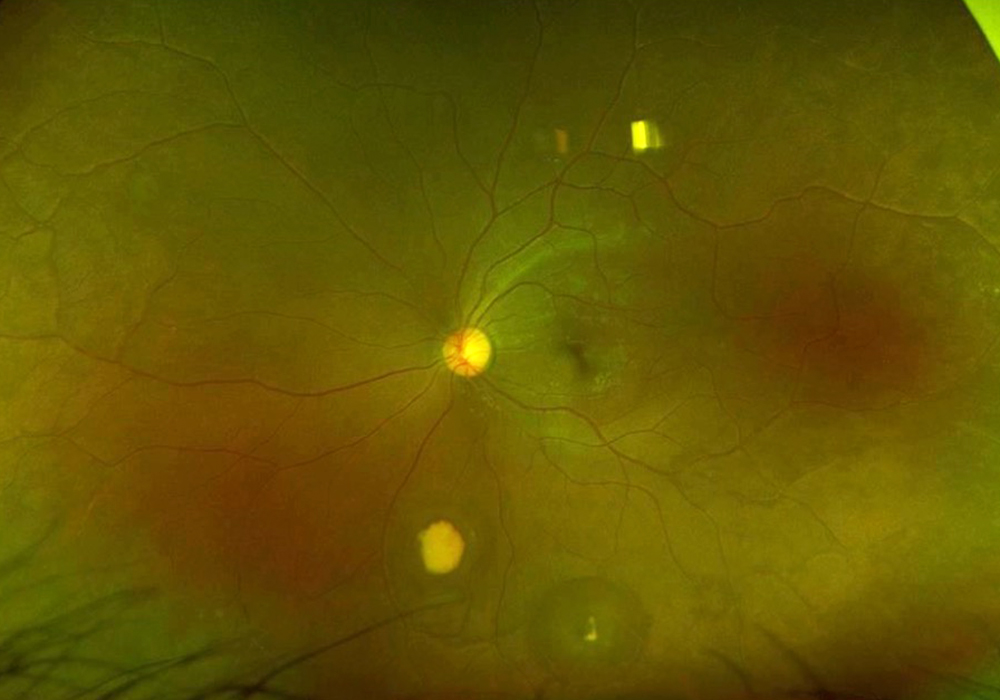
Figure 4B: Wide-field color photograph of the left eye. Note the yellowish area of old hemorrhage inferiorly
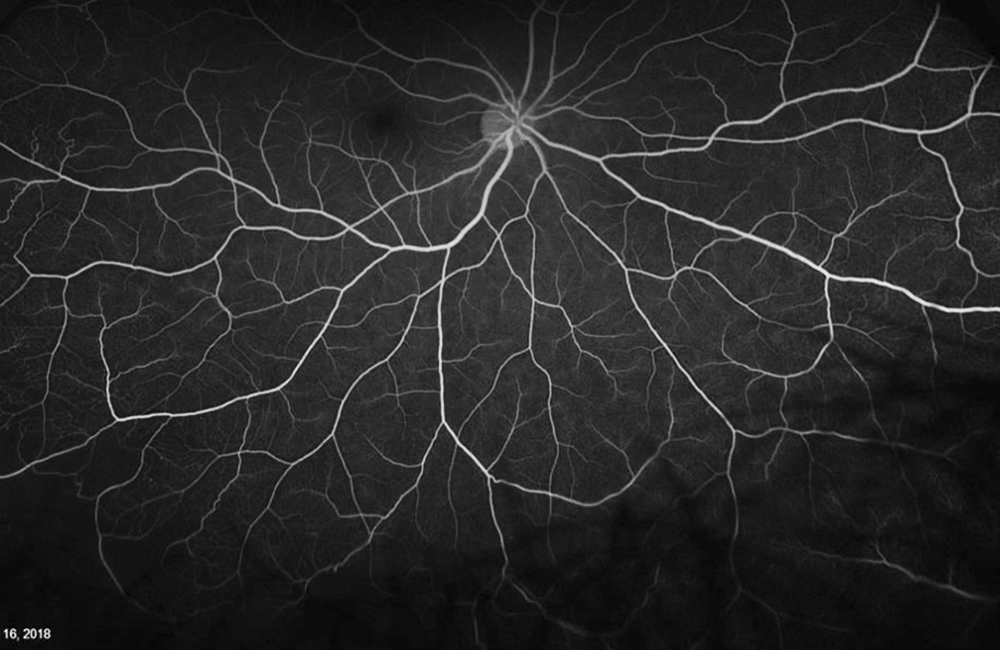
Figure 5A: Wide-field color angiogram of the right eye.
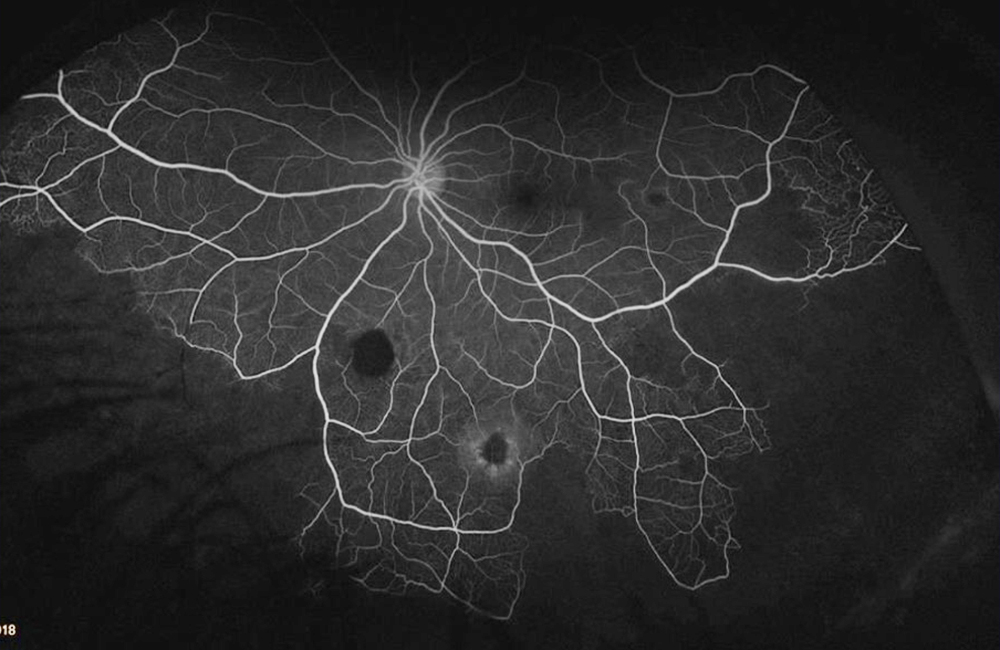
Figure 5B: Wide-field angiogram of the left eye. Note the areas of nonperfusion. The area of old hemorrhage inferiorly shows hypofluorescence due to blocking .
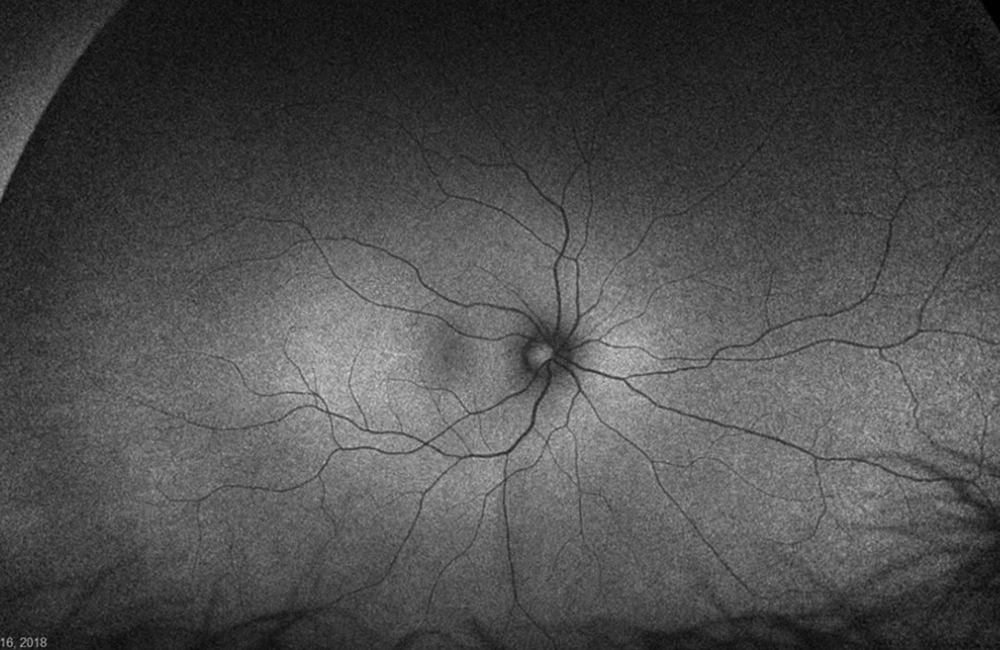
Figure 6A: Wide-field autofluorescence of the right eye. No abnormal autofluorescence is seen.
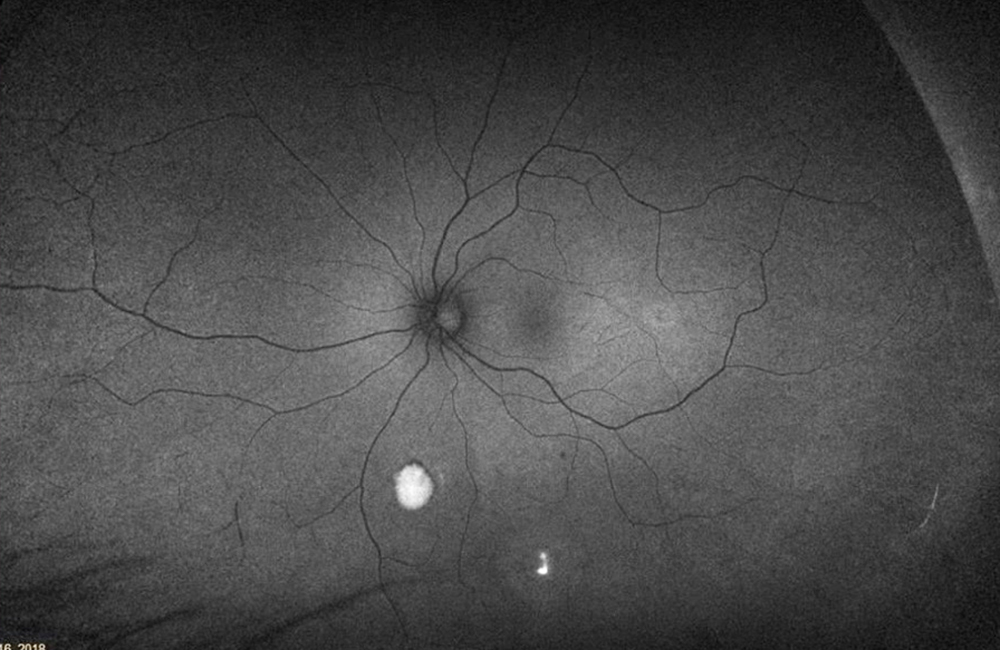
Figure 6B:Wide-field autofluorescence of the left eye. Hyper autofluorescence is seen inferiorly in the areas of old hemorrhage.
This patient has non-proliferative sickle cell retinopathy with peripheral non-perfusion in both eyes and macular ischemia in the left eye. The patient’s left eye vision has fluctuated between 20/125 and 20/40 over the last 5 years. The patient has been on hydroxyurea and Endari (L-glutamine oral powder) for the last four years, with no known hospitalizations during this period.
Discussion
Sickle cell retinopathy is an ocular manifestation of sickle cell disease. Sickle cell disease (SCD) is a hemoglobinopathy that results from mutation of a single nucleotide (GAG to GTG, resulting in an amino acid switch from glutamic acid to valine) in the beta subunit of hemoglobin, a protein in red blood cells that transports oxygen.1 A heterozygous mutation results in sickle cell trait (AS) and homozygous mutation results in sickle cell disease (SS). Other mutations in the beta subunit can result in hemoglobin SC disease or sickle thalassemia (SThal).1 African Americans are most commonly affected, with an incidence of sickle cell trait (AS) of 8% and sickle cell disease (SS) of 0.4%.2
Normal red blood cells can pass through smaller caliber blood vessels easily. However, red blood cells in SCD patients sickle under hypoxic conditions leading to decreased vascular flow and occlusion.3 This manifests as characteristic damage in various structures of the eye, including the retina. Sickle cell retinopathy can be classified as non-proliferative or proliferative. Non-proliferative sickle retinopathy occurs secondary to vaso-occlusion and localized ischemia. A salmon patch hemorrhage is an occlusion and hemorrhage of a superficial retinal blood vessel in the periphery. Initially appearing red, the lesion becomes salmon colored due to dehemoglobinization of the hemorrhage. As the hemorrhage is resorbed, refractile deposits (hemosiderin and macrophages) can be noted under the internal limiting membrane. Black sunburst spots occur due to migration and proliferation of RPE cells in response to damage caused by hemorrhage. Occlusion in the choroidal circulation can cause breaks in the Bruch’s membrane, known as angioid streaks. Proliferative sickle retinopathy occurs secondary to chronic ischemia and upregulation of vascular growth factors. The Goldberg classification consist of 5 stages of proliferative sickle retinopathy: Stage 1 peripheral arterial occlusion, Stage 2 peripheral arteriovenous anastomoses, Stage 3 sea fan neovascularization, Stage 4 vitreous hemorrhage, Stage 5 tractional retinal detachment.4 Of note, incidence of proliferative retinopathy is highest in patient with SC or SThal (33% and 14%, respectively) compared to SS (3%).2
Macular ischemia is in the spectrum of non-proliferative sickle retinopathy and can cause a significant burden of vision loss, as seen in our patient.5 OCT and OCTA based findings can help us detect more subtle macular perfusion abnormalities that often precede the more severe and chronic peripheral retinal findings in proliferative sickle retinopathy. Three key findings are noteworthy. The first is an enlarged foveal avascular zone. In a study by Roemer et al, OCTA demonstrated a larger nonflow area and lower capillary density in sickle eyes compared to healthy controls.6-9 The second is thinning of the inner more than outer retinal structures in the temporal macula. It is hypothesized that the inner retinal structures are more affected due to the smaller caliber of retinal capillaries supplying the inner retina, compared to the choriocapillaris supplying the outer retina. Also, the temporal macula is thought to be more affected than the nasal as it represents a watershed area between the superior and inferior vascular arcades. More severe retinal thinning has been associated with age, stage of retinopathy, and the SS subtype of disease.10-12 Of note, Alport’s syndrome is another disease involving the retina that can classically present with temporal retinal thinning. Lastly, though our patient did not demonstrate this finding, foveal splaying can occur. This refers to the thinning of the parafoveal region and may represent either the residua of, or precursor to, macular infarctions.13