Case #171
Presented by Lawrence Chan, MD


Presented by Lawrence Chan, MD

A 14-year-old male is referred for decreased vision in both eyes.
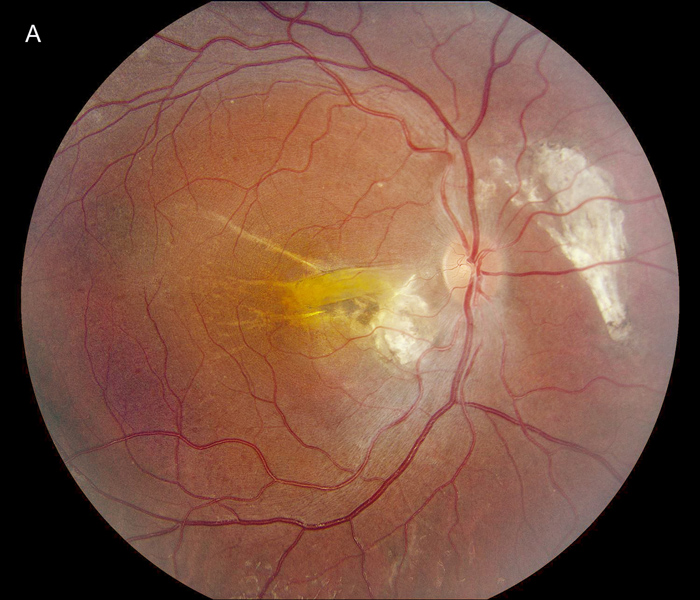
Figure 1A: Color photo of the right eye. Note chroioretinal scarring nasal to the nerve and nasal to the fovea. A retinal fold is present in the macula.
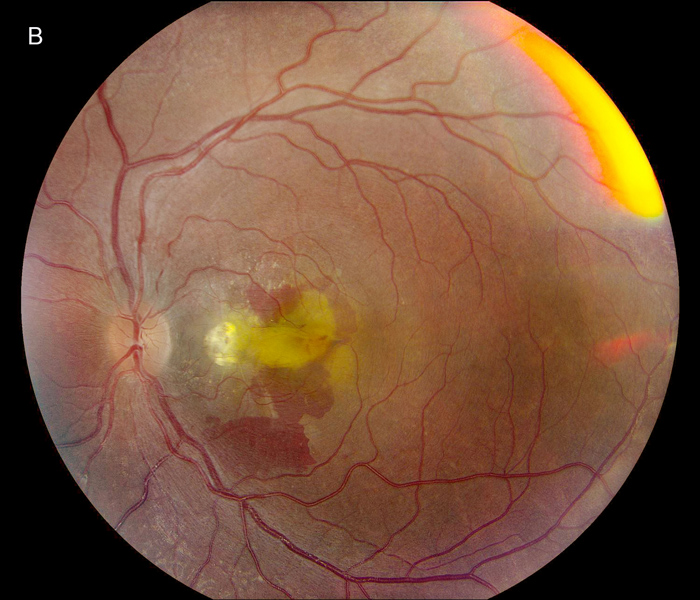
Figure 1B:Color photo of the left eye. Note the presence of a retinal fold, similar to the right eye. Similar chorioretinal scarring is present nasal to the fovea. Significant subretinal hemorrhage is present.
On presentation, our patient reported a decrease in central vision in the left eye over the past few weeks. He had also been having longstanding night blindness and difficulties adapting to dark environments. His past ocular history was notable for bilateral retinal folds, microphthalmia, hyperopia, esotropia, and retinal dystrophy.
At 13 months of age, he was noted to have difficulty tracking with his right eye. He was found to have a papillomacular fold OD and was prescribed corrective lenses for hyperopia, along with patching and atropine treatment for possible amblyopia OD. At age 3, he was found to have subretinal fibrosis OD and a yellow peripapillary fibrotic lesion encroaching on the fovea OS. Ultrasonography revealed bilaterally short globes with thickening of the posterior sclera. Electroretinography showed a reduction in rod>cone function. Genetic testing revealed a single mutation in the NR2E3 gene (c. 119-2A>C; IVS1-2A>C), which has been reported to be a pathogenic mutation seen in autosomal recessive enhanced S-cone syndrome (PMID 10655056). A second NR2E3 mutation was not identified at that time.
His past medical history was notable for resolved iron deficiency. He was the product of an egg donor and surrogate mother. He did not have a history of prematurity. There was no past surgical history. He was not on any medications other than multivitamin supplements. He did not have any known drug allergies. Smoking, alcohol, and illicit substance use history was negative. There was no history of vision issues in his biological parents. No family history of consanguinity was noted. Review of systems was negative.
The patient's best corrected Snellen visual acuity at distance was 20/250 OD and 20/250 pinhole 20/200 OS (from baseline of 20/125 OD and 20/30 OS one year ago). Intraocular pressures were 15 OU. Anterior segment exam was appropriate for age. The posterior segment exam in the right eye was notable for a papillomacular fold with adjacent linear/patchy chorioretinal scarring and hyperpigmentation, as well as an area of subretinal fibrosis just nasal to the disc (Figure 1A). The left eye posterior segment exam showed a less prominent retinal fold with a subfoveal choroidal neovascular membrane (CNVM), adjacent subretinal hemorrhages, and chorioretinal scarring just nasal to the CNVM (Figure 1B). OCT macula of the right eye revealed subretinal fibrosis, loss of the outer retinal layers, and trace intraretinal cysts (Figure 2A). In the left eye, there was subretinal hyperreflective material consistent with subretinal hemorrhage, an overlying outer retinal tubulation, and disorganization of the retinal layers (Figure 2B). Fundus autofluorescence images OU revealed symmetric patterns of pericentral hypoautofluorescence along the vascular arcades with inferonasal sparing, hypoautofluorescence at areas of fibrosis, and hyperautofluorescent flecks peripherally (Figures 3A and 3B). Fluorescein angiography (FA) OD showed window defects and staining consistent with the areas of chorioretinal atrophy and fibrosis, respectively (Figure 4A); FA OS revealed active leakage of the CNVM with blockage from the subretinal hemorrhage and staining of the nasal parafoveal fibrosis (Figure 4B).
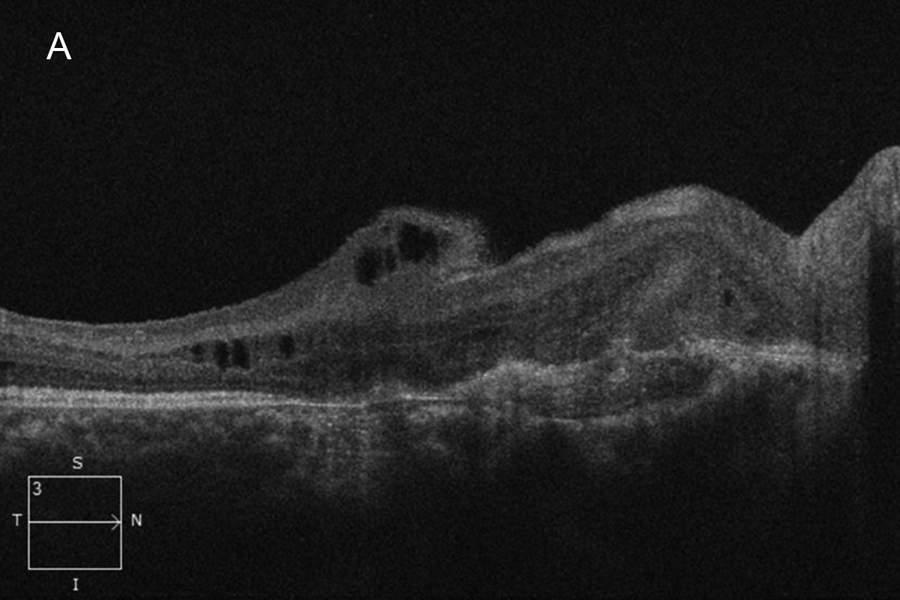
Figure 2A:OCT-SD scab oif the right macula. Subretinal fibrosis is present and disorganized retinal layers. Retinal thinning is noted temporally.
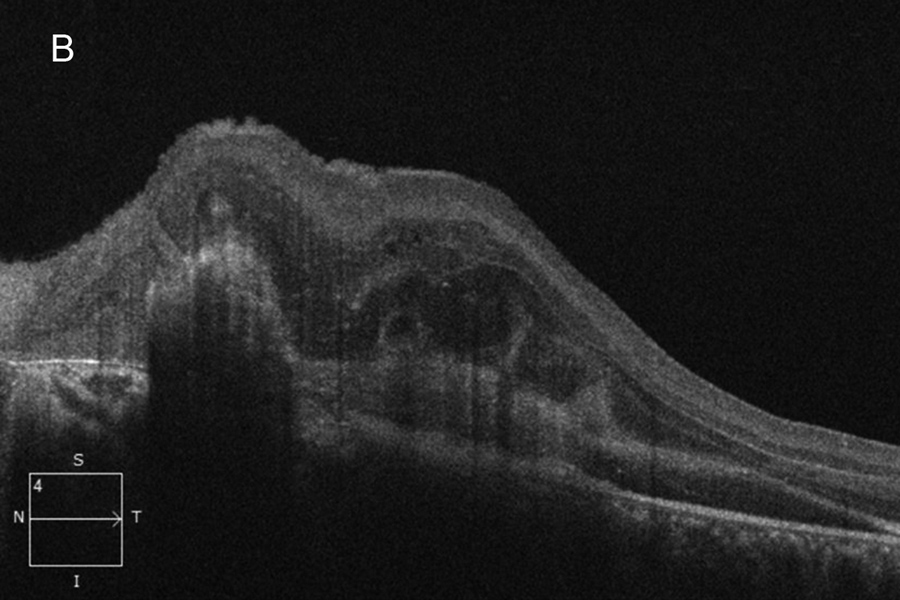
Figure 2B:OCT-SD scan of the left macula. Subretinal hyperreflective material is present due to the subretinal hemorrhage. Intraretinal edema and thickening is present in addition to chorioretinal scarring.
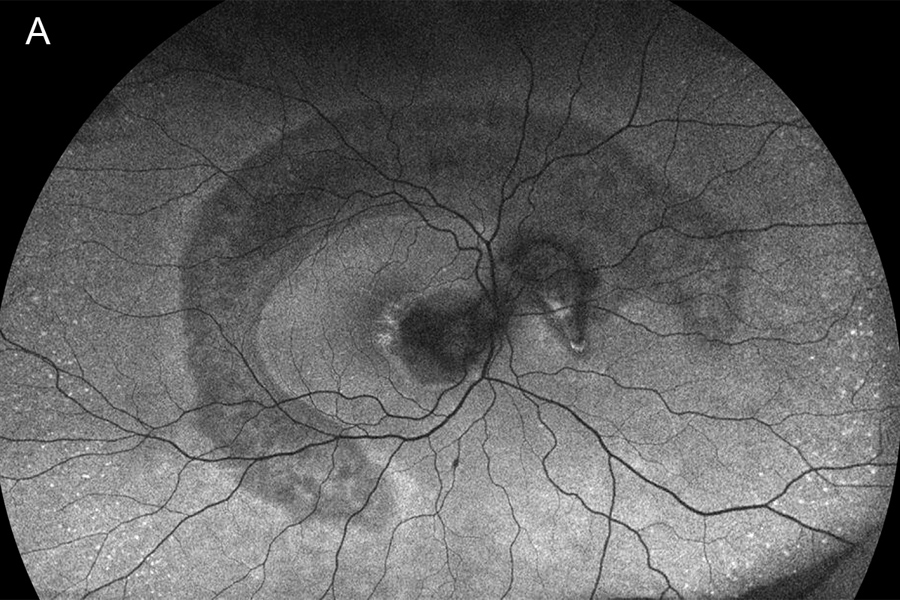
Figure 3A:Wide-field fundus autofluorescence of the right eye. Note the large crescent shaped area of hypoautofluorescense arcing around the macula and into the nasal retina. More significant hypofluorescence is present in the macula.
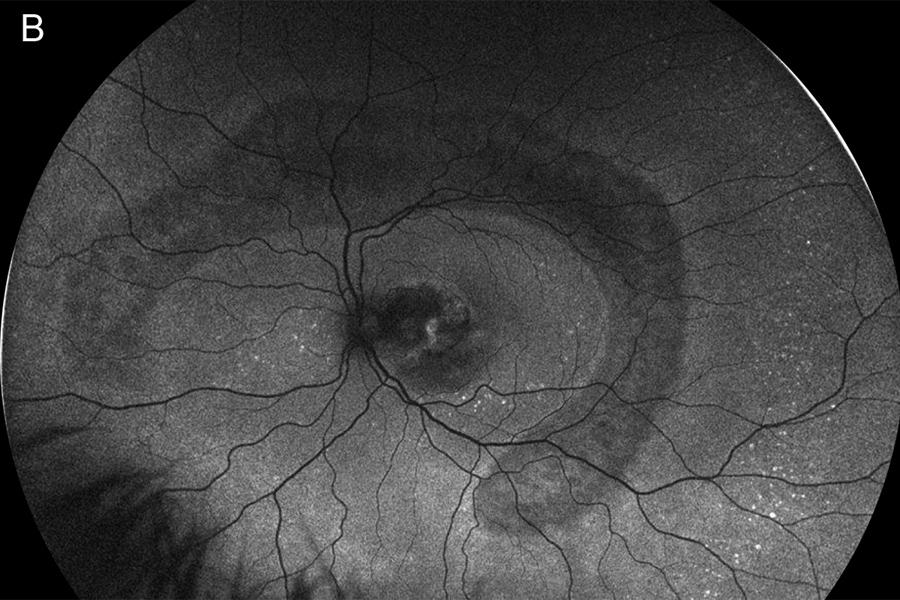
Figure 3B: Wide-field autofluorescence of the left eye. A similar pattern is present as was noted in the left eye.
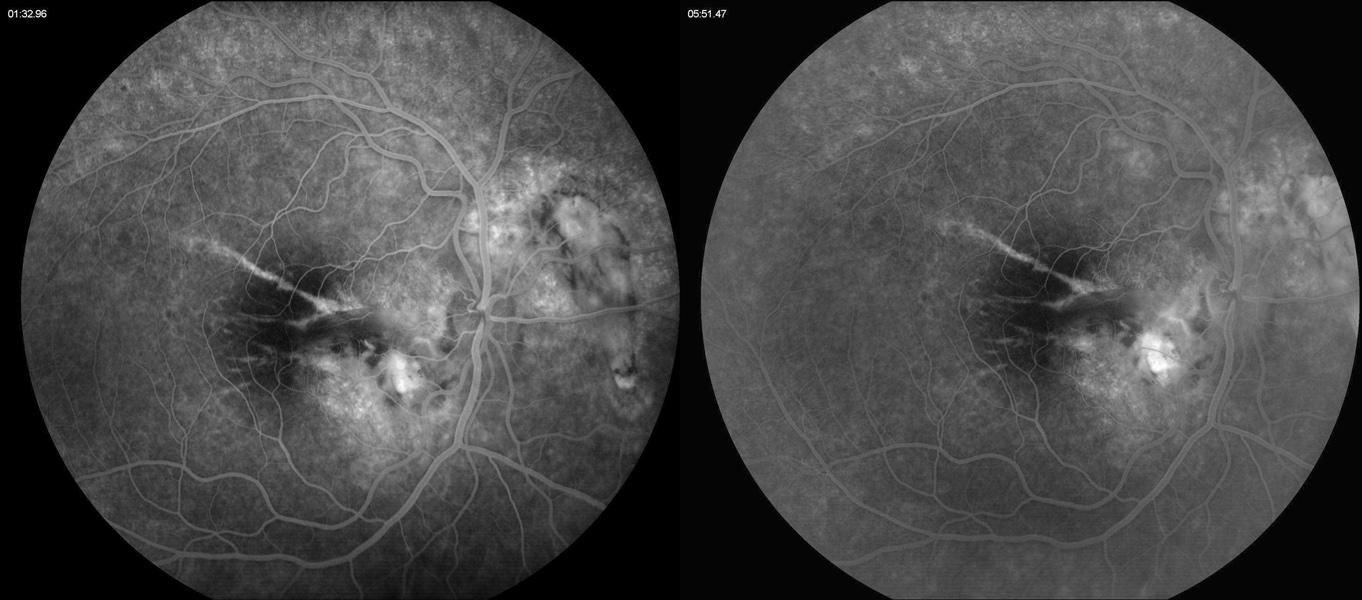
Figure 4A:Fluorescein angiogram of the right eye. Note the areas of hyperfluorescence due to RPE scarring and atrophy. Some late staining is present in the area of the subretinal fibrosis and chorioretinal scarring.
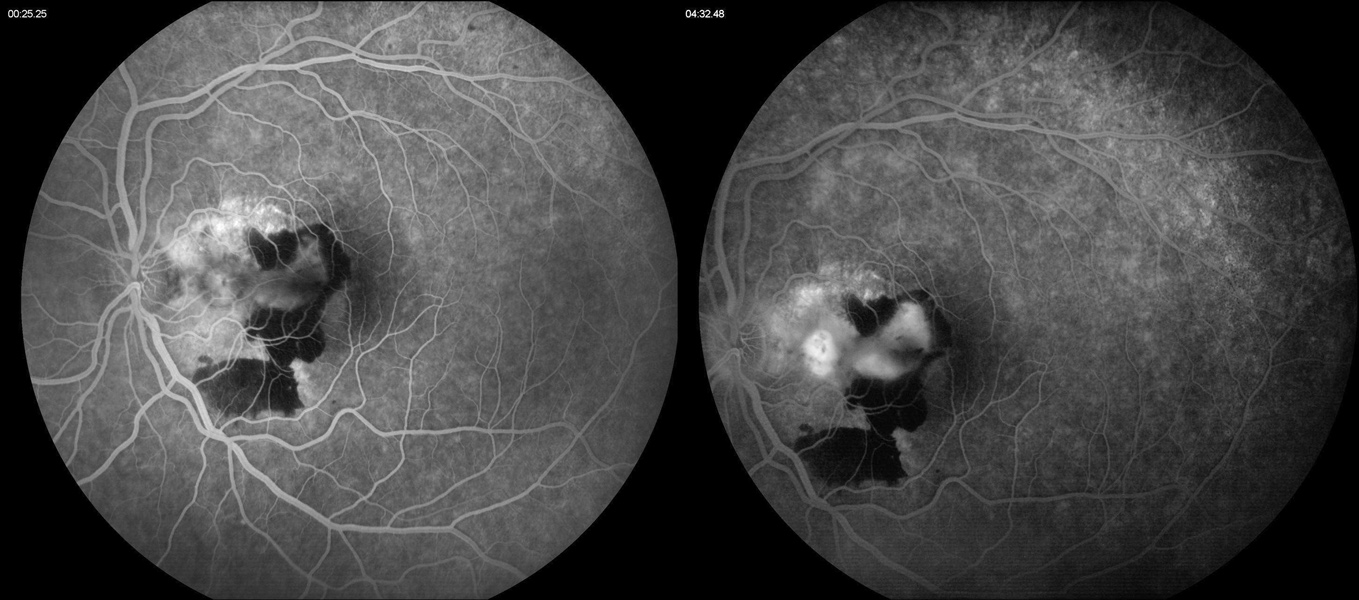
Figure 3B:Fluorescein angiogram of the left eye. Note the area of leakage in the macula centered around the area of blocked fluorescence due to the subretinal hemorrhage.
Differential Diagnosis
Additional Case History and Patient Course
Given the findings of active CNV in the left eye, a trial of a series of monthly intravitreal aflibercept injections was recommended. After each injection, there was regression of the exudation and subretinal hemorrhage with gradual improvement in the VA OS from 20/250 to 20/50 after four injections (Figures 5 and 6). His clinical exam has remained stable for at least three months after completing the series of intravitreal injections.
Repeat genetic testing was performed. In addition to the previously identified pathogenic mutation on the NR2E3 gene, an NR2E3 variant of uncertain significance (c. 349G>A [p. Ala117Thr]) was found on an opposite chromosome.
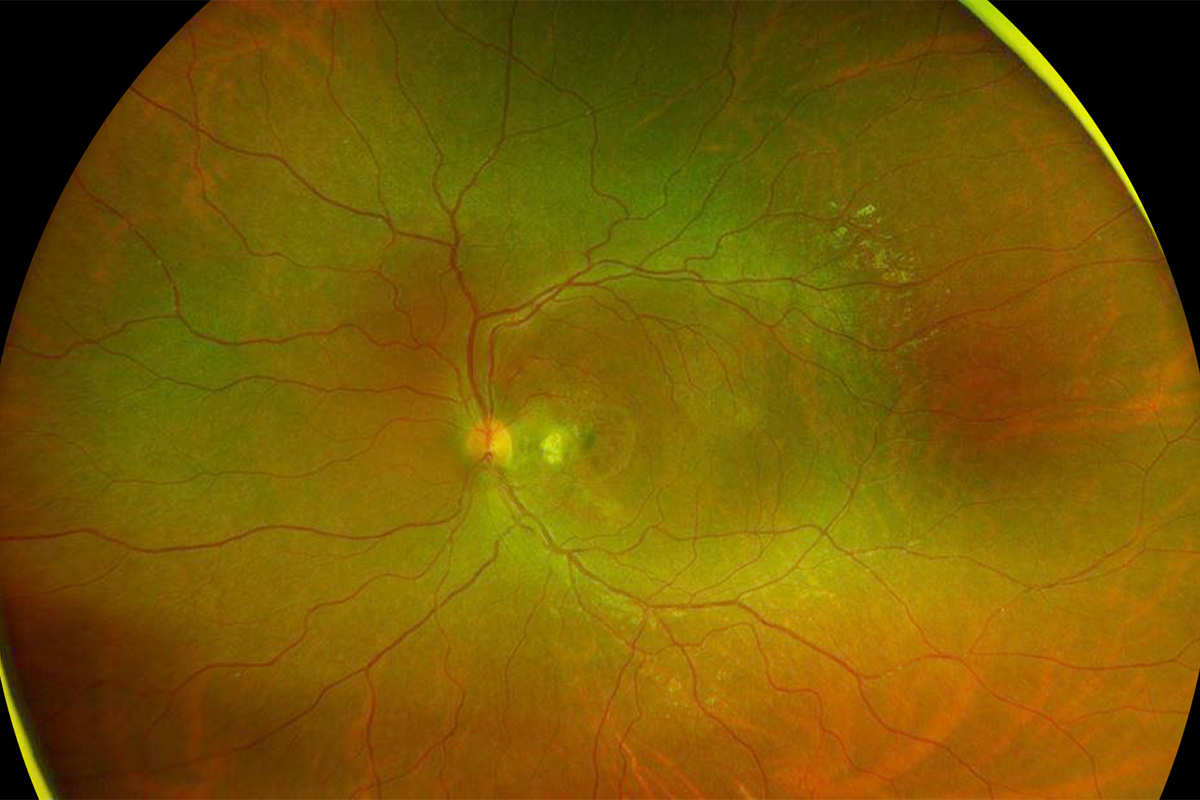
Figure 5: Wide-field color photo of the left eye. Following 4 monthly antiVEGF injections, the choroidal neovascularization has regressed and the subretinal hemorrhage resorbed.
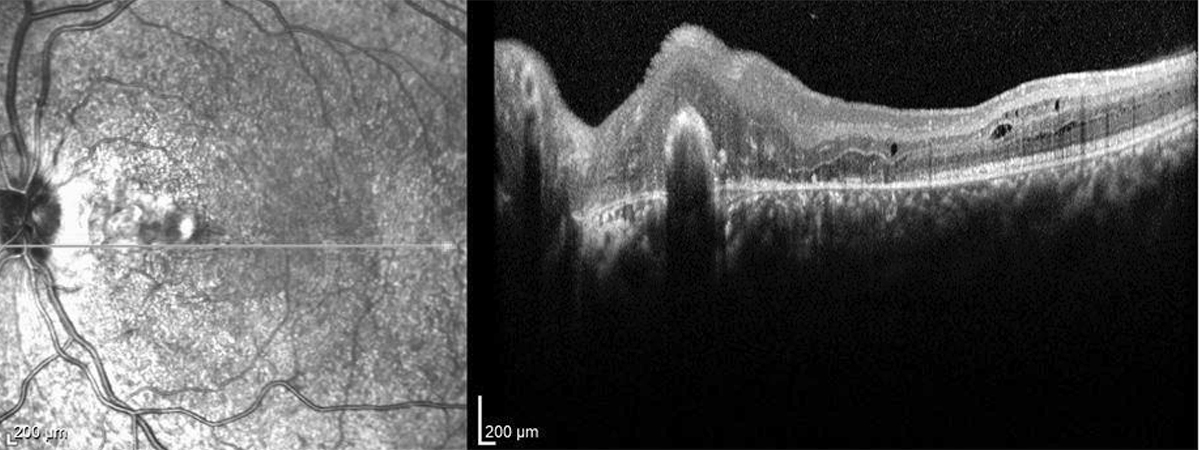
Figure 6:OCT-SD of the left macula. The subretinal hemorrhage, intraretinal edema/thickening has resolved. The chorioretinal scarring is noted nasally.
Discussion
Enhanced S-cone syndrome (ESCS) is a slowly progressive, autosomal recessive inherited retinal degeneration caused mainly by mutations in the nuclear receptor class 2, subfamily E, member 3 (NR2E3) gene.1 The gene is expressed in the outer nuclear layer of the retina and acts to promote rod photoreceptor differentiation while repressing short wavelength-sensitive S-cone gene expression. Thus, loss of proper NR2E3 function results in a lack of rod photoreceptor development and an overabundance of S-cones being produced.2 This is responsible for the clinical findings seen in patients with ESCS, namely early onset of night blindness, decreased central visual acuity, hemeralopia, and increased sensitivity to blue light.3
Electroretinography (ERG) is crucial in the diagnosis of ESCS with pathognomonic findings: 1) extinguished rod response, 2) similarly reduced and delayed waveforms under both scotopic and photopic conditions, 3) delayed and decreased amplitudes on 30Hz flicker responses, and 4) supernormal response to blue light (short wavelength) on an orange background to suppress red and green cones.4 OCT scans may show areas of foveal schisis, subretinal hyperreflective deposits, RPE atrophy, and subretinal fibrosis due to choroidal neovascularization.5 Fundus autofluorescence typically reveals hypoautofluorescence near or beyond the arcades due to photoreceptor loss. Hyperautofluorescence may be seen due to accumulation of lipofuscin secondary to RPE-photoreceptor dysfunction.4
Various phenotypic manifestations of ESCS have been described in the literature. Fundus findings include nummular RPE pigment clumping along the vascular arcades and midperiphery, macular or peripheral retinoschisis, chorioretinal atrophy, macular subretinal fibrosis, torpedo-like lesions of chorioretinal atrophy/RPE depigmentation with hyperpigmented margins, and intraretinal yellow dots.5 Although uncommon, choroidal neovascularization has also been seen in pediatric cases of ECSC with reports of good response to treatment with anti-vascular endothelial growth factor (anti-VEGF) injections.6,7 Involution of prior untreated CNV membranes can lead to the development of subretinal fibrosis.7 Although the exact mechanisms behind CNV formation in ESCS have not been fully elucidated, some authors theorize that photoreceptor dysfunction, RPE changes, and choriocapillaris damage result in areas that are susceptible to neovascular growth of choroidal vessels.7 Others hypothesize that the relative hypoxia from the higher oxygen demands of the increased number of cones that characterize ESCS causes upregulation of VEGF and subsequent neovascularization.8